DailyMed – ALTOPREV- lovastatin tablet, extended release – Inergency
[ad_1]
Primary Hyperlipidemia in Adults
Altoprev has been shown to reduce Total-C, LDL-C, and TG and increase HDL-C in patients with hyperlipidemia. Near maximal response was observed after four weeks of treatment and the response was maintained with continuation of therapy for up to 6 months.
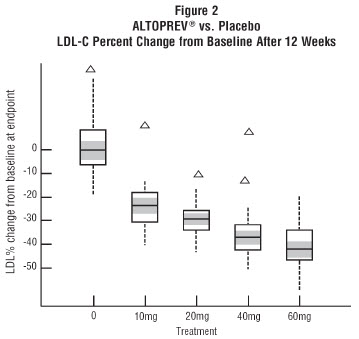
In a 12-week, multicenter, placebo-controlled, double-blind, trial in adult males and females 21 to 70 years of age with primary hyperlipidemia, once daily administration of Altoprev 20 to 60 mg in the evening was compared to placebo. Altoprev produced dose related reductions in LDL-C and Total-C. The lipid changes with Altoprev treatment in this trial, from baseline to endpoint, are displayed in Table 7.
The range of LDL-C responses is represented graphically in the following figure (Figure 1):
Figure 1 Altoprev vs. Placebo LDL-C Percent Change from Baseline After 12 Weeks
The distribution of LDL-C responses is represented graphically by the boxplots in Figure 1. The bottom line of the box represents the 25th percentile and the top line, the 75th percentile. The horizontal line in the box represents the median and the gray area is the 95% confidence interval for the median. The range of responses is depicted by the tails and outliers.
Expanded Clinical Evaluation of Lovastatin Immediate-Release (EXCEL) Study
Lovastatin immediate-release was compared to placebo in 8,245 patients with hyperlipidemia [Total-C 240 to 300mg/dL, LDL-C >160 mg/dL] in the randomized, double-blind, parallel, 48-week EXCEL trial. All changes in the lipid measurements (see Table 8) observed in patients administered lovastatin immediate-release were dose-related and significantly different from placebo (p≤0.001). These results were sustained throughout the trial.
Heterozygous Familial Hypercholesterolemia in Adults
Lovastatin immediate-release has been shown to be effective in reducing Total-C and LDL-C in heterozygous familial and non-familial forms of primary hypercholesterolemia. A response was seen within 2 weeks, and the maximum response occurred within 4-6 weeks. The response was maintained during continuation of therapy.
Prevention of Coronary Heart Disease
The Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS), a double-blind, randomized, placebo-controlled, primary prevention trial, demonstrated that treatment with lovastatin immediate-release decreased the rate of acute major coronary events (composite endpoint of myocardial infarction, unstable angina, and sudden cardiac death) compared with placebo during a median of 5.1 years of follow-up. Participants were males (ages 45 to 73) and females (ages 55 to 73) without symptomatic cardiovascular disease with average to moderately elevated Total-C and LDL-C, below average HDL-C, and who were at high risk based on elevated Total-C/HDL-C. In addition to age, 63% of the participants had at least one other risk factor (baseline HDL-C <35 mg/dL, hypertension, family history, smoking and diabetes).
AFCAPS/TexCAPS enrolled 6,605 participants (5,608 males, 997 females) based on the following lipid entry criteria: Total-C range of 180 to 264 mg/dL, LDL-C range of 130 to 190 mg/dL, HDL-C of ≤45 mg/dL for males and ≤47 mg/dL for females, and TG of ≤400 mg/dL. Participants were treated with standard care, including diet, and either lovastatin immediate-release 20 mg to 40 mg daily (n= 3,304) or placebo (n= 3,301). Approximately 50% of the participants treated with lovastatin immediate-release were titrated to 40 mg daily when their LDL-C remained >110 mg/dL at the 20-mg starting dose.
Lovastatin immediate-release reduced the risk of a first acute major coronary event, the primary efficacy endpoint, by 37% (lovastatin immediate-release 3.5%, placebo 5.5%; p<0.001; Figure 2). A first acute major coronary event was defined as myocardial infarction (54 participants on lovastatin immediate-release, 94 on placebo) or unstable angina (54 vs. 80) or sudden cardiac death (8 vs. 9). Furthermore, among the secondary endpoints, lovastatin immediate-release reduced the risk of unstable angina by 32% (1.8% vs. 2.6%; p=0.023), of myocardial infarction by 40% (1.7% vs. 2.9%; p=0.002), and of undergoing coronary revascularization procedures (e.g., coronary artery bypass grafting or percutaneous transluminal coronary angioplasty) by 33% (3.2% vs. 4.8%; p=0.001). Trends in risk reduction associated with treatment with lovastatin immediate-release were consistent across males and females, smokers and non-smokers, hypertensives and non-hypertensives, and older and younger participants. Participants with ≥2 risk factors had risk reductions (RR) in both acute major coronary events (RR 43%) and coronary revascularization procedures (RR 37%). Because there were too few events among those participants with age as their only risk factor in this study, the effect of lovastatin immediate-release on outcomes can not be adequately assessed in this subgroup.
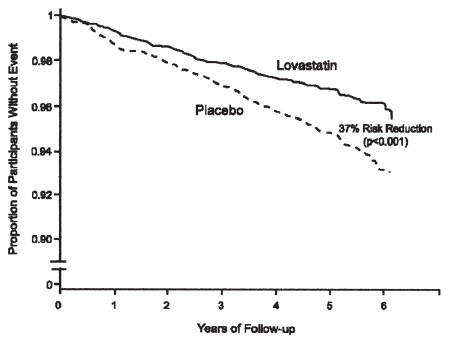
Figure 2 Acute Major Coronary Events (Primary Endpoint)
Atherosclerosis
In the Canadian Coronary Atherosclerosis Intervention Trial (CCAIT), the effect of therapy with lovastatin immediate-release on coronary atherosclerosis was assessed by coronary angiography in hyperlipidemic patients. In this randomized, double-blind, controlled clinical trial, patients were treated with conventional measures (usually diet and 325 mg of aspirin every other day) and either lovastatin immediate-release 20 mg to 80 mg daily or placebo (the 80 mg dose is not approved for Altoprev [see Dosage and Administration (2.2)]). Angiograms were evaluated at baseline and at two years by computerized quantitative coronary angiography (QCA). Lovastatin immediate-release significantly slowed the progression of lesions as measured by the mean change per-patient in minimum lumen diameter (the primary endpoint) and percent diameter stenosis, and decreased the proportions of patients categorized with disease progression (33% vs. 50%) and with new lesions (16% vs. 32%).
[ad_2]