How blocking a neural receptor responsible for addiction could reduce alcohol use
[ad_1]
Scripps Research scientists have found that LY2444296—a compound that selectively blocks the kappa opioid receptor (KOP)—may reduce drinking in cases of alcohol dependence in animal studies. The findings, which were published in Scientific Reports, could eventually inform new treatment options for people who experience alcohol use disorder (AUD).
“Compounds designed to block the KOP selectively are very promising because this receptor is involved in a lot of mental illnesses, such as anxiety and depression,” says Rémi Martin-Fardon, Ph.D., an associate professor in the Department of Molecular Medicine. “The KOP system is also important in alcohol use disorder, so the idea is if it’s targeted and blocked, you can stop alcohol abuse.”
The KOP system controls brain circuits that affect a range of neurological processes, including addiction, emotion, pain and reward, and reward-seeking. Both acute and chronic exposure to alcohol negatively affects this system, according to the study’s first author, Francisco Flores-Ramirez, Ph.D., a postdoctoral fellow at Scripps Research.
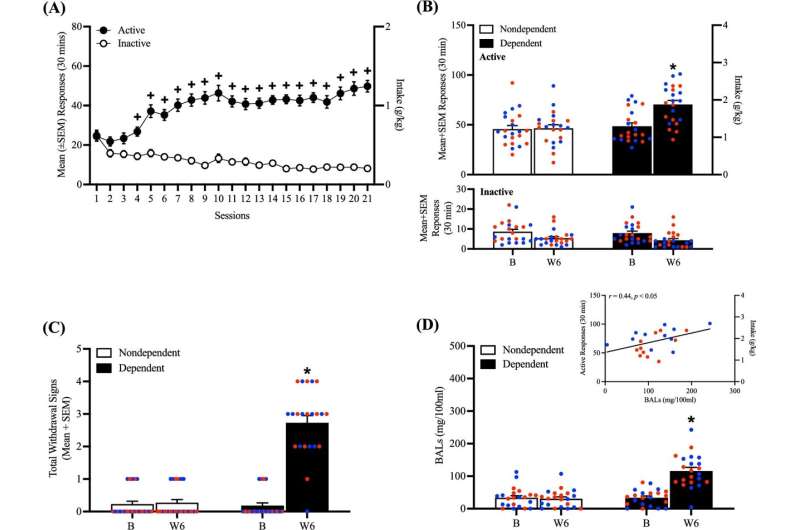
For their study, Martin-Fardon and Flores-Ramirez sought to find out whether orally administering LY2444296 could decrease alcohol consumption in rats that formed alcohol dependency. The aim was to mitigate withdrawal symptoms, which would hypothetically lead to reduced alcohol intake.
Once rats received LY2444296 at doses as low as 3 mg per kg following 8 hours of abstinence—when acute withdrawal symptoms typically start— withdrawal signs and alcohol consumption tapered down significantly. The researchers also determined that LY2444296 may be innocuous, as it had neither a positive nor negative effect on rats without alcohol dependency.
Martin-Fardon and his team didn’t expect LY2444296 to reduce withdrawal signs after only 8 hours of alcohol abstinence because earlier studies showed that other compounds capable of binding to the KOP had no effect on alcohol withdrawal. The scientists don’t yet know why LY2444296 was effective in the present study, and they plan to investigate further.
“People drink to get rid of the sensations of withdrawal,” Martin-Fardon says. He added that withdrawal is associated with physical pain and that oftentimes, “the only thing that can fix the problem is to have a drink.” But if LY2444296 is taken before withdrawal symptoms begin, “you can decrease the symptoms, so you feel better and drink less.”
Still, the question remains: which specific parts of the brain are best targeted to mitigate withdrawal symptoms? Next on their agenda, Martin-Fardon and Flores-Ramirez hope to determine whether LY24444296 can block the effects of stress and other cues that can trigger alcohol relapse.
“We’re also interested in what brain regions are changing as a function of alcohol dependence,” Flores-Ramirez says. “Maybe we could target them to see if the compound could reverse both drinking and relapse behavior.”
More information:
Francisco J. Flores-Ramirez et al, LY2444296, a κ-opioid receptor antagonist, selectively reduces alcohol drinking in male and female Wistar rats with a history of alcohol dependence, Scientific Reports (2024). DOI: 10.1038/s41598-024-56500-9
Citation:
How blocking a neural receptor responsible for addiction could reduce alcohol use (2024, March 29)
retrieved 5 April 2024
from https://medicalxpress.com/news/2024-03-blocking-neural-receptor-responsible-addiction.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
[ad_2]
Source link