Presence and Distribution of Sun-Blocking Agents in the Danube River: Implications for Aquatic Ecosystem Health
[ad_1]
1. Introduction
In this study, we aim to establish the concentration levels of UVFs in the Danube River and assess their ecological implications on aquatic ecosystems. The identification of UVFs in surface water and sediment samples will provide valuable insights into the environmental risks associated with these compounds. By conducting an ecological risk assessment, we can better understand the potential effects of UVFs on aquatic organisms and improve environmental stewardship practices in Romania and globally. This study introduces a novel approach to investigating organic UVFs in the Danube River, focusing on the utilization of a rapid and sensitive LC-MS/MS method. The method allows for a quick elution time of only 12 min, enabling efficient analysis of UVF compounds in surface water and sediment samples. The method’s sensitivity is demonstrated by the low limits of quantification (LOQs), ranging from 0.1 to 0.69 ng/L for surface water samples and 0.21 to 0.79 ng/g dry weight for sediment samples. These results showcase the advancement in analytical techniques employed in this study, providing detailed insights into the occurrence and levels of UVF contaminants in the Danube River ecosystem. The combination of the fast elution time and high sensitivity of the LC-MS/MS method contributes significantly to the innovation and robustness of this research, offering valuable contributions to the field of environmental monitoring and risk assessment related to UVFs in aquatic environments.
2. Materials and Methods
2.1. Study Area and Sample Collection
Surface water and sediment samples were collected along the Danube River from five sampling points in September 2020: Bazias (S1, N 44.8166, E 21.3887), Eselnita (S2, N 44.6817, E 22.3559), Gruia (S3, N 44.2630, E 22.6894), Zimnicea (S4, N 43.6437, E 25.3325), and Giurgiu (S5, N 43.8862, E 25.9907). During the sampling process, 2 L of surface water was gathered from specific locations using clean glass containers. These containers were first rinsed with ultra-pure water and then rinsed with the sample water before obtaining the samples. Sediment samples were collected using a stainless-steel device, and the top 10 cm of sediment was specifically targeted for sampling. The samples were then placed in glass jars (previously cleaned with acetone) and stored at −4 °C until sample preparation.
2.2. Chemicals
2.3. Sample Preparation
2.4. LC-MS/MS Analysis
2.5. Total Organic Carbon (TOC) Analysis
TOC analysis was conducted using a TOC-LCPN instrument featuring an infrared detector obtained from Shimadzu in Japan. The TOC values were determined by combusting the samples at 720 °C in a synthetic air flow. Each sample was measured in duplicate.
2.6. Environmental Risk Assessment
2.7. Quality Assurance and Quality Control
3. Results
3.1. Occurrence of Sun-Blocking Agents in Surface Water
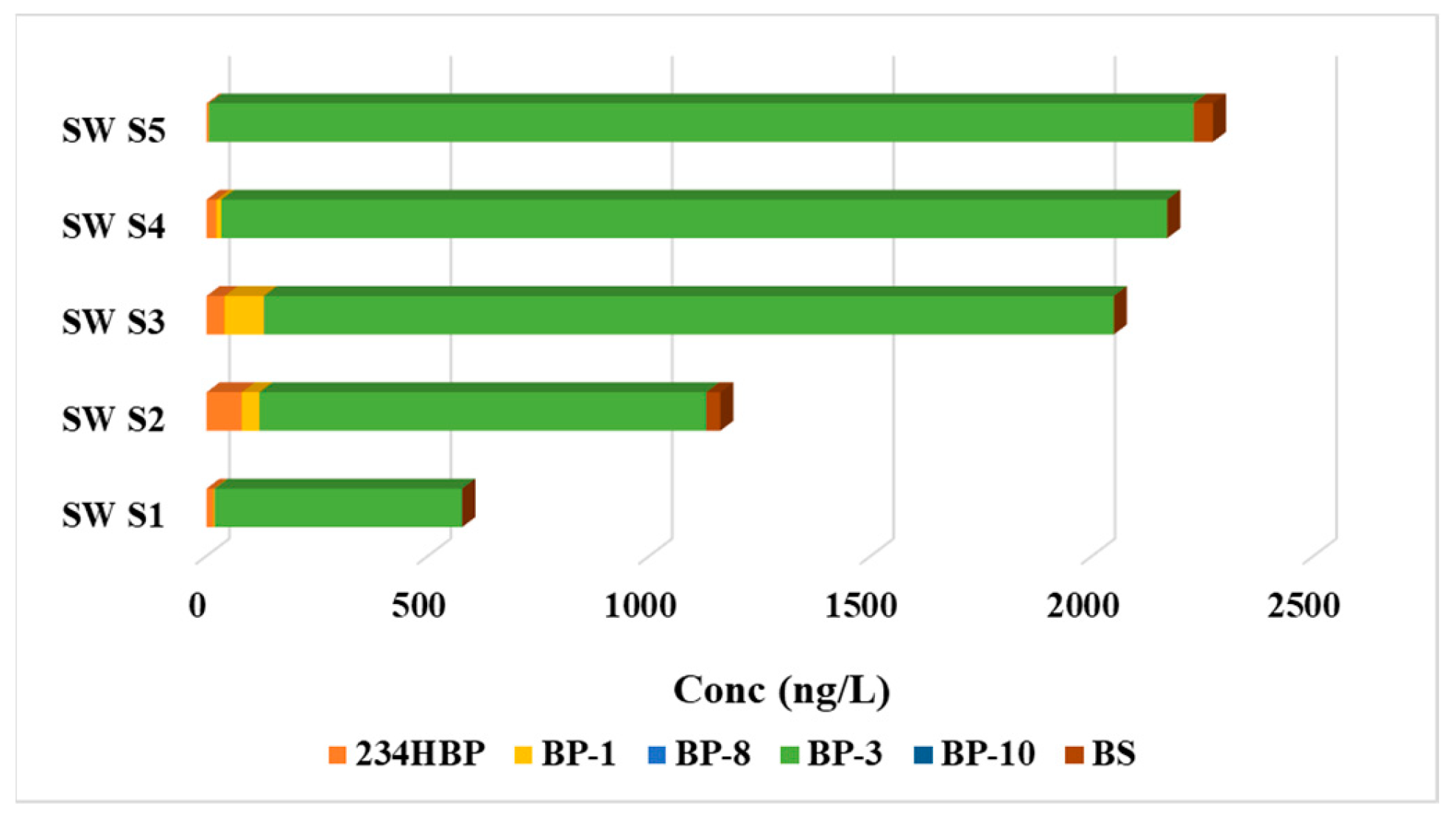
It is worth noting that the samples were collected in September, marking the end of the sunbathing season for European tourists. This timing is significant as it captures the potential impact of UV filters used during the peak sun exposure period in the region. The detection of UVFs in the samples collected at this time provides insight into the persistence of these compounds in the aquatic environment, even after the sunbathing season has concluded. This information is valuable for understanding the environmental implications of UV filter contamination in the Danube River, particularly following periods of heightened sun exposure and UV filter usage by tourists in the area.
3.2. Occurrence of Sun-Blocking Agents in Sediment
3.3. Distribution Pattern of Sun-Blocking Agents in Surface Water and Sediment
Distinct patterns of organic UV filter (UVFs) contamination were identified through the examination of surface water and sediment samples. Surface water samples were primarily contaminated by BP-3, 234HBP, and BP-1, while sediment samples were dominated by more hydrophobic UVFs with higher partition coefficients, such as BS and BP-3. The differences in the types of contaminants found in surface water and sediment indicate varying sources and behavior of UVFs in aquatic environments.
3.4. Correlation with TOC
The complexity of the aquatic environment, including the presence of various organic compounds and the dynamic interactions between different phases, can further complicate the relationship between TOC content and the UVF amount. The partitioning behavior of UVFs and their interactions with other organic compounds may vary based on factors such as environmental conditions and the specific characteristics of the contaminants.
The lack of correlation between TOC content and the UVF amount highlights the intricate nature of organic compound distribution and dynamics in aquatic systems. Additional research and thorough investigations are required to clarify the factors that impact the occurrence and behavior of UVFs concerning TOC content in both surface water and sediment samples.
3.5. Potential Environmental Risk for Individual UVFs in Surface Water
The findings of the environmental risk assessment emphasize the significance of assessing the potential effects of UVFs on aquatic ecosystems. The high RQ values for BP-1 and BP-3 highlight the need for further monitoring and research to better understand the ecological implications of these compounds. Mitigation measures may be necessary to reduce the risk posed by these UVFs to aquatic species and overall ecosystem health.
The results of the risk assessment highlight the crucial need to take into account both the concentration levels of UVFs in the environment and their toxicological impacts on aquatic organisms. Continued research and monitoring efforts are essential to assess and mitigate the potential risks associated with UVF contamination in aquatic ecosystems.
4. Discussion
The results of this study shed light on the presence of six organic UV filters (UVFs) in surface water and sediment samples collected along the Danube River in Romania. The detection of BP-3, BP-1, and 234HBP in the surface water samples at elevated concentrations indicates widespread contamination of UVFs in the aquatic environment. The dominance of BP-3 in surface water samples can be attributed to its common use in personal care products, highlighting the potential sources of contamination along the river course. The significant concentrations of 234HBP and BP-1 suggest the environmental degradation of BP-3, leading to the formation of degradation products in the aquatic ecosystem. The detection of BS in specific surface water samples underscores the presence of salicylate derivatives in the river, with potential effects on aquatic organisms.
In sediment samples, the predominance of BS and BP-3 indicates the accumulation of more hydrophobic UVFs with higher partition coefficients compared to water samples. The escalating contamination levels of UV filters along the Danube River from Romania to the Black Sea suggest a continuous buildup of pollutants throughout the river course. The presence of 234HBP and BP-2 in sediment samples further highlights the transformation of parent compounds into degradation products in the sediment environment. The differences in the types of contaminants found in surface water and sediment samples indicate varying sources and behaviors of UVFs in aquatic environments.
The Pearson correlation analysis revealed significant positive and negative correlations among UVFs in surface water and sediment samples, suggesting potential degradation pathways and transformation processes among these compounds. The lack of correlation between total organic carbon (TOC) content and the UVF amount in both surface water and sediment samples can be attributed to the complex interactions between different organic compounds in aquatic systems, influencing the distribution and fate of UVFs.
The environmental risk assessment based on short-term toxicity data and maximum concentration levels of UVFs in surface water samples indicated varying levels of risk for aquatic organisms. While 234HBP and BS posed minimal risk to Daphnia magna, BP-1 and BP-3 showed a potential environmental risk, particularly for fish and Daphnia magna. The high risk quotients (RQs) for BP-1 and BP-3 underscore the need for continued monitoring and research to mitigate the ecological impact of these compounds on aquatic ecosystems.
This study highlights the importance of assessing the presence, distribution, and ecological implications of UVFs in aquatic environments. The findings underscore the need for ongoing monitoring and evaluation of UVF contamination to protect aquatic species and ecosystem health. Mitigation measures may be necessary to reduce the risks posed by UVFs and safeguard water quality in the Danube River and beyond. Further research is essential to elucidate the complex interactions between UVFs and other organic compounds in aquatic systems and to mitigate potential threats to aquatic ecosystems.
5. Conclusions
This study provides valuable insights into the presence and distribution of UV filters (UVFs) in the Danube River, highlighting the extensive contamination of these compounds in both surface water and sediment samples. The concentration range observed in the analyzed samples varied between n.d. (not detected) and 2224 ng/L in surface water samples, and between n.d. and 244 ng/g d.w. in sediment samples. The most commonly detected compounds in all surface water samples were 234HBP, BP-1, and BP-3, with BP-3 being found at levels of up to 2244 ng/L. In sediment samples, BP-3 and BS were the predominant compounds identified in all samples, with BP-3 reaching levels of up to 129 ng/g d.w. and BS up to 244 ng/g d.w. The prevalence of UVFs, such as 234HBP, BP-1, BP-3, and BS, underscores the potential environmental risks associated with these compounds in this aquatic ecosystem.
The correlation analysis conducted in this study suggests potential common sources and biodegradation pathways for UVFs in the dynamic aquatic environment of the Danube River. The observed correlations between different UVFs indicate complex interactions and transformation processes among these compounds, emphasizing the interconnected nature of UVF contamination in the study area.
The environmental risk assessment revealed varying risk levels for different UVFs, with compounds like BP-1 and BP-3 posing significant risks to aquatic species. These findings underscore the importance of considering both the presence of UVFs and their toxicological effects on organisms when assessing environmental risks.
While this study provides valuable insights, it is essential to acknowledge its limitations. This study focused on a specific region of the Danube River and a limited set of UVFs, so the findings may not be generalizable to other regions or compounds. Additionally, the short-term toxicity data used for the risk assessment may not fully capture the long-term effects of UVFs on aquatic ecosystems.
Moving forward, future studies should expand the scope of research to encompass a broader range of UVFs and locations along the Danube River. Investigating the sources, fate, and impacts of UVFs in more detail will inform effective management strategies and conservation efforts. Continued monitoring and research are crucial to mitigate potential risks and safeguard the health of aquatic ecosystems in the Danube River and beyond.
Supplementary Materials
Author Contributions
Conceptualization, F.L.C. and L.F.P.; methodology, F.P. and I.P.; software, F.P.; I.P. and I.A.C.; validation, F.L.C. and L.F.P.; formal analysis, F.P. and I.P.; investigation, F.P.; I.P. and I.A.C.; resources, I.A.C.; data curation, F.P. and I.P.; writing—original draft preparation, F.P. and I.P.; writing—review and editing, F.L.C. and L.F.P.; visualization, I.A.C.; supervision, F.L.C. and L.F.P.; project administration, F.L.C.; funding acquisition, F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Research Program “Nucleu” through contract no. 20N/2019, Project code PN 19 04 01 01.
Data Availability Statement
Acknowledgments
We acknowledge the use of AI technology for its support in improving English grammar in the final stage of our manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorganica Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.F.; Lam, T.K.; Leung, K.S.Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Wang, C.; Xie, T.; Xu, R.; Lin, J.; Li, L. Simultaneous determination of ultraviolet absorbers and antibacterial agents in textiles by ultra-high-performance liquid chromatography/orbitrap high resolution mass spectrometry. World J. Eng. 2017, 5, 1–18. [Google Scholar] [CrossRef]
- European Union. EU Cosmetics Directive 76/768/ECC Consolidated Version 2004; European Union: Maastricht, The Netherlands, 2004. [Google Scholar]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. Advances in analytical methods and occurrence of organic UV-filters in the environment—A review. Sci. Total Environ. 2015, 526, 278–311. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; He, Y.; Gin, K.Y.H. Occurrence and fate of benzophenone-type UV filters in aquatic environments: A review. Environ. Sci. Water Res. Technol. 2019, 5, 209–223. [Google Scholar] [CrossRef]
- You, L.; Nguyen, V.T.; Pal, A.; Chen, H.; He, Y.; Reinhard, M.; Gin, K.Y.H. Investigation of pharmaceuticals, personal care products and endocrine disrupting chemicals in a tropical urban catchment and the influence of environmental factors. Sci. Total Environ. 2015, 536, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Corada-Fernández, C.; Candela, L.; Torres-Fuentes, N.; Pintado-Herrera, M.G.; Paniw, M.; González-Mazo, E. Effects of extreme rainfall events on the distribution of selected emerging contaminants in surface and groundwater: The Guadalete River basin (SW, Spain). Sci. Total Environ. 2017, 605–606, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, F.L.; Paun, I.; Pirvu, F.; Iancu, V.I.; Galaon, T. Distribution, removal efficiencies and environmental risk assessment of benzophenone and salicylate UV filters in WWTPs and surface waters from Romania. New J. Chem. 2021, 45, 2478–2487. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Pirvu, F.; Paun, I. Investigation of endocrine disruptor pollutants and their metabolites along the Romanian Black Sea Coast: Occurrence, distribution and risk assessment. Environ. Toxicol. Pharmacol. 2021, 8, 103673. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Leung, H.W.; Wai, T.C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef]
- Rodríguez, A.S.; Sanz, M.R.; Rodríguez, J.R.B. Chemosphere Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands). Chemosphere 2015, 131, 85–90. [Google Scholar] [CrossRef]
- Herrera, M.G.P.; Martín, P.A.L. Fate and Behavior of UV Filters in the Marine. In Sunscreens in Coastal Ecosystems: Occurrence, Behavior, Effect and Risk the Handbook of Environmental Chemistry; Blasco, J., Tovar, A., Sánchez, D., Eds.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Astel, A.; Stec, M.; Rykowska, I. Occurrence and Distribution of UV Filters in Beach Sediments of the Southern Baltic Sea Coast. Water 2020, 12, 3024. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Molina-Molina, J.M.; Zafra-Gómez, A.; Ballesteros, O.; Navalóna, A.; Real, M.; Sáenz, J.M.; Fernández, M.F.; Olea, N. Simultaneous determination of the UV-filters benzyl salicylate, phenyl salicylate, octyl salicylate, homosalate, 3-(4-methylbenzylidene) camphor and 3-benzylidene camphor in human placental tissue by LC–MS/MS. Assess-ment of their in vitro endocrine activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 936, 80–87. [Google Scholar] [CrossRef]
- Frikeche, J.; Couteau, C.; Roussakis, C.; Coiffar, L.J.M. Research on the immunosuppressive activity of ingredients contained in sunscreens. Arch. Dermatol. 2015, 307, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Kojima, H.; Takeuchi, S.; Uramaru, N.; Sanoh, S.; Sugihara, K.; Kitamura, S.; Ohta, S. Metabolism of UV-filter benzophenone-3 by rat and human liver microsomes and its effect on endocrine-disrupting activity. Toxicol. Appl. Pharmacol. 2015, 282, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Sechman, A.; Batoryna, M.; Antos, P.A.; Hrabia, A. Effects of PCB 126 and PCB 153 on secretion of steroid hormones and mRNA expression of steroidogenic genes (STAR, HSD3B, CYP19A1) and estrogen receptors (ERα, ERβ) in PR hierarchical chicken ovarian follicles. Toxicol. Lett. 2016, 264, 29–37. [Google Scholar] [CrossRef]
- Fent, K.; Kunz, P.Y.; Zenker, A.; Rapp, M. A tentative environmental risk assessment of the UV-filters 3-(4-methylbenzylidene-camphor), 2-ethyl-hexyl-4-trimethoxycinnamate, benzophenone-3, benzophenone-4 and 3-benzylidene camphor. Mar. Environ. Res. 2010, 69, S4–S6. [Google Scholar] [CrossRef]
- Ozáez, I.; Martínez-Guitarte, J.L.; Morcillo, G. The UV filter benzophenone 3 (BP-3) activates hormonal genes mimicking the action of ecdysone and alters embryo development in the insect Chironomus riparius (Diptera). Environ. Pollut. 2014, 192, 19–26. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Mastroianni, N.; Díaz-Cruz, M.S.; Barcelóa, D. Fully automated determination of nine ultraviolet filters and transformation products in natural waters and wastewaters by on-line solid phase extraction–liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1294, 106–116. [Google Scholar] [CrossRef]
- Wu, M.H.; Xie, D.G.; Xu, G.; Sun, R.; Xia, X.Y.; Liu, W.L.; Tang, L. Benzophenone-type UV filters in surface waters: An assessment of profiles and ecological risks in Shanghai, China. Ecotoxicol. Environ. Saf. 2017, 141, 235–241. [Google Scholar] [CrossRef]
- Serra-Roig, M.P.; Jurado, A.; Díaz-Cruz, M.S.; Vázquez-Suñé, E.; Pujades, E.; Barceló, D. Occurrence, fate and risk assessment of personal care products in river–groundwater in-terface. Sci. Total Environ. 2016, 568, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Blondel, C.; Khelalfa, F.; Reynaud, S.; Fauvelle, F.; Raveton, M. Effect of organochlorine pesticides exposure on the maize root metabolome assessed using high-resolution magic-angle spinning 1H NMR spectroscopy. Environ. Pollut. 2014, 214, 539–548. [Google Scholar] [CrossRef]
- Kunisue, T.; Chen, Z.; Buck Louis, G.M.; Sundaram, R.; Hediger, M.L.; Sun, L.; Kannan, K. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ. Sci. Technol. 2012, 46, 4624–4632. [Google Scholar] [CrossRef]
- Grabicova, K.; Fedorova, G.; Burkina, V.; Steinbach, C.; Schmidt-Posthaus, H.; Zlabek, V.; Kroupova, H.K.; Grabic, R.; Randak, T. Presence of UV filters in surface water and the effects of phenylbenzimidazole sulfonic acid on rainbow trout (Oncorhynchus mykiss) following a chronic toxicity test. Ecotoxicol. Environ. Saf. 2013, 96, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Chen, H.C.; Ding, W.H. Ultrasound-assisted dispersive liquid–liquid micro-extraction plus simultaneous silylation for rapid determination of salicylate and benzo-phenone-type ultraviolet filters in aqueous samples. J. Chromatogr. A 2013, 1302, 20–27. [Google Scholar] [CrossRef]
- Wu, M.H.; Li, J.; Xu, G.; Ma, L.D.; Li, J.J.; Li, J.S.; Tang, L. Pollution patterns and underlying relationships of benzophenone-type UV-filters in wastewater treatment plants and their receiving surface water. Ecotoxicol. Environ. Saf. 2018, 152, 98–103. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; He, K.; Gonsior, M.L.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; Wang, C.; Luc, J.; Chang, Y.P.; Chen, W.; Li, X.; Lara-Martín, P.A. Distribution, mass inventories, and ecological risk assessment of legacy and emerging contaminants in sediments from the Pearl River Estuary in China. J. Hazard. Mater 2017, 323, 128–138. [Google Scholar] [CrossRef]
- Vila, M.; Llompart, M.; Garcia-Jares, C.; Homem, V.; Dagnac, T. Development and optimization of a solid-phase microextraction gas chromatography–tandem mass spectrometry methodology to analyse ultraviolet filters in beach sand. J. Chromatogr. A 2018, 1564, 59–68. [Google Scholar] [CrossRef]
- Apel, C.; Tang, J.; Ebbinghaus, R. Environmental occurrence and distribution of organic UV stabilizers and UV filters in the sediment of Chinese Bohai and Yellow Seas. Environ. Pollut. 2018, 235, 85–94. [Google Scholar] [CrossRef]
- Kameda, Y.; Kimura, K.; Miyazaki, M. Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes. Environ. Pollut. 2011, 159, 1570–1576. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Leung, H.W.; Kwana, B.K.Y.; Nga, K.Y.; Yamashita, N.; Taniyasu, S.; Lama, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in marine sediments in Hong Kong and Japan. J. Hazard. Mater. 2015, 292, 180–187. [Google Scholar] [CrossRef]
Figure 1.
The total amount of UVFs determined in surface water samples.
Figure 1.
The total amount of UVFs determined in surface water samples.
Figure 2.
The total amount of UVFs determined in sediment samples.
Figure 2.
The total amount of UVFs determined in sediment samples.
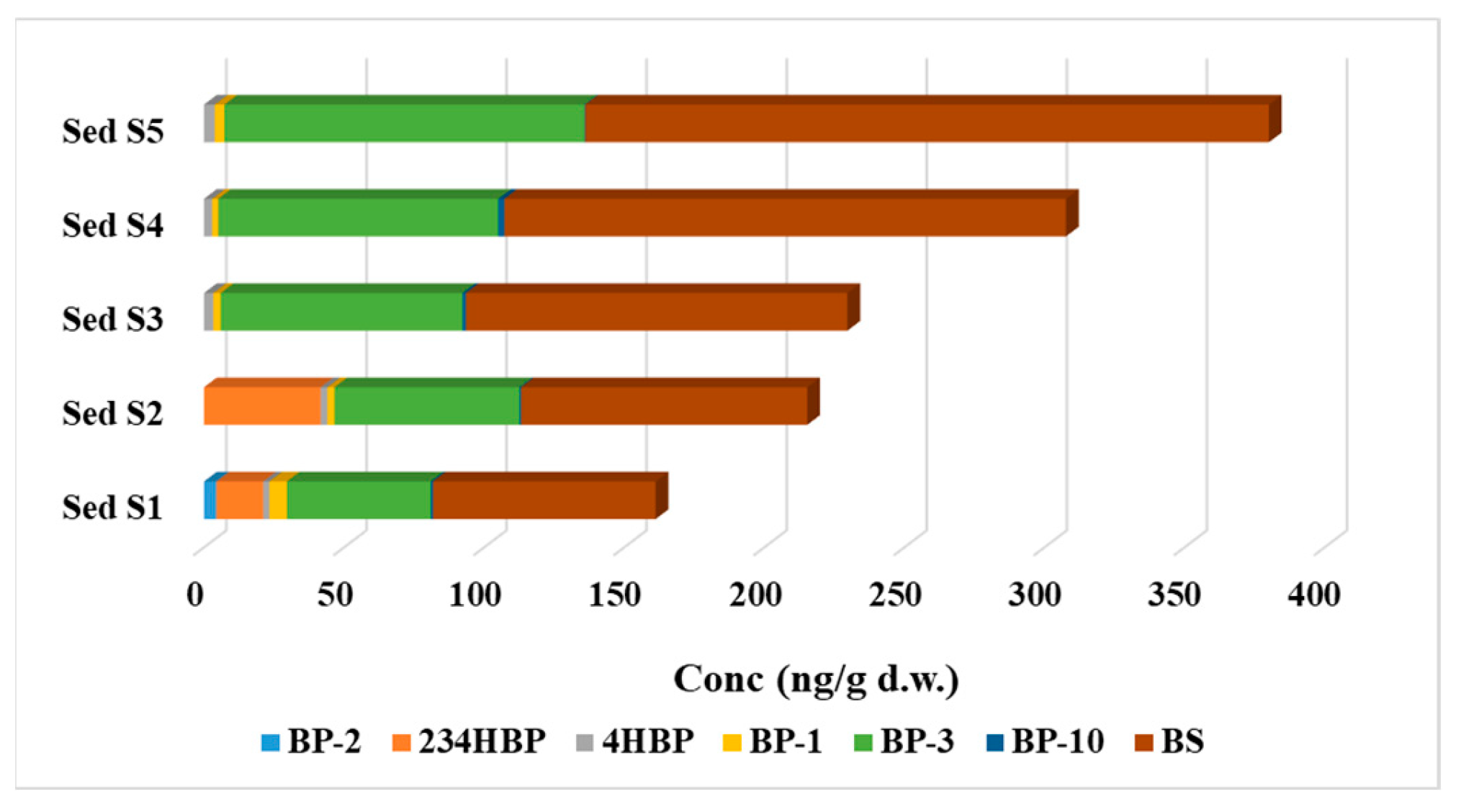
Table 1.
Pearson correlation coefficients of UVFs in surface water samples.
Table 1.
Pearson correlation coefficients of UVFs in surface water samples.
| SW (n = 5) | |||||||
|---|---|---|---|---|---|---|---|
| Analytes | 4HBP | BP-1 | BP-8 | BP-3 | BP-10 | BS | |
| 234HBP | Pearson Corr. | 0.205 | 0.900 | −0.112 | 0.867 | 0.100 | −0.224 |
| p-value | 0.7406 | 0.037 | 0.858 | 0.052 | 0.873 | 0.718 | |
| 4HBP | Pearson Corr. | 0.564 | 0.459 | −0.051 | 0.154 | −0.860 | |
| p-value | 0.322 | 0.437 | 0.935 | 0.805 | 0.061 | ||
| BP-1 | Pearson Corr. | 0.112 | −0.800 | 0.200 | −0.447 | ||
| p-value | 0.858 | 0.624 | 0.747 | 0.450 | |||
| BP-8 | Pearson Corr. | −0.671 | 0.894 | −0.625 | |||
| p-value | 0.215 | 0.041 | 0.260 | ||||
| BP-3 | Pearson Corr. | −0.800 | 0.447 | ||||
| p-value | 0.044 | 0.450 | |||||
| BP-10 | Pearson Corr. | −0.335 | |||||
| p-value | 0.417 | ||||||
Table 2.
Pearson correlation coefficients of UVFs in sediment samples.
Table 2.
Pearson correlation coefficients of UVFs in sediment samples.
| SED (n = 5) | ||||||
|---|---|---|---|---|---|---|
| Analytes | 4HBP | BP-1 | BP-3 | BP-10 | BS | |
| 234THBP | Pearson Corr. | −0.783 | 0.112 | 0.619 | −0.335 | −0.783 |
| p-value | 0.017 | 0.858 | 0.118 | 0.581 | 0.118 | |
| 4HBP | Pearson Corr. | −0.100 | 0.900 | −0.200 | 0.900 | |
| p-value | 0.873 | 0.037 | 0.747 | 0.037 | ||
| BP-1 | Pearson Corr. | −0.300 | −0.500 | −0.300 | ||
| p-value | 0.624 | 0.391 | 0.624 | |||
| BP-3 | Pearson Corr. | −0.100 | 1.000 | |||
| p-value | 0.873 | 0.000 | ||||
| BP-10 | Pearson Corr. | −0.100 | ||||
| p-value | 0.873 | |||||
Table 3.
The environmental risk (ER) assessment of organic UV filters in surface water.
Table 3.
The environmental risk (ER) assessment of organic UV filters in surface water.
| Analytes | Toxicity | Species | NOEC (ng/L) | MEC (ng/L) | RQ | ER |
|---|---|---|---|---|---|---|
| 234THBP | Chronic | Daphnia magna | 29,400,000 b | 80 | 0.0003 | It is unlikely to pose a risk |
| 4HBP | Chronic | – | – | 2 | – | – |
| BP-1 | Chronic | Fish | 4919 a | 89 | 1.81 | High risk |
| BP-3 | Chronic | Daphnia magna | 180,000 b | 2224 | 1.24 | High risk |
| Chronic | Fish | 3900 b | 2224 | 57.0 | High risk | |
| BP-10 | Chronic | – | – | 1 | – | – |
| BS | Chronic | Daphnia magna | 894,000 b | 43 | 0.005 | It is unlikely to pose a risk |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
[ad_2]