Biomedicines | Free Full-Text | Activation of MAP Kinase Pathway by Polyisoprenylated Cysteinyl Amide Inhibitors Causes Apoptosis and Disrupts Breast Cancer Cell Invasion
[ad_1]
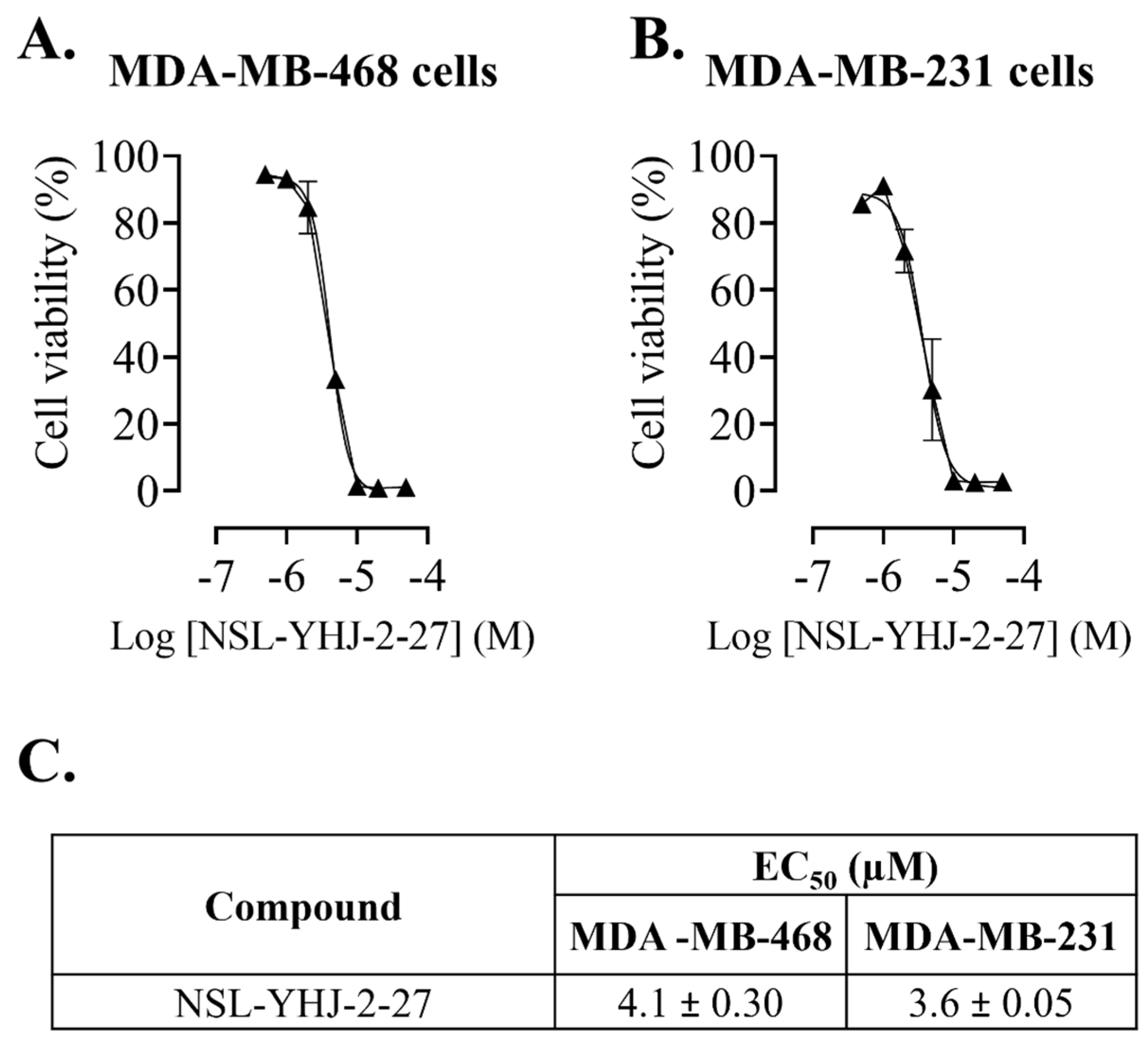
Figure 1.
NSL-YHJ-2-27 suppresses the viability of breast cancer cells. After treatment of cells with the indicated concentrations of NSL-YHJ-2-27 (0, 0.5, 1, 2, 5, 10, 20, and 50 µM), resazurin reduction assay was performed to determine the viability of the cells. (A,B) Cell viabilities were plotted against varying concentrations of NSL-YHJ-2-27 expressed as the percentage of fluorescence intensity compared to the controls. (C) EC50 values for the effects of the treatments on cell viability.
Figure 1.
NSL-YHJ-2-27 suppresses the viability of breast cancer cells. After treatment of cells with the indicated concentrations of NSL-YHJ-2-27 (0, 0.5, 1, 2, 5, 10, 20, and 50 µM), resazurin reduction assay was performed to determine the viability of the cells. (A,B) Cell viabilities were plotted against varying concentrations of NSL-YHJ-2-27 expressed as the percentage of fluorescence intensity compared to the controls. (C) EC50 values for the effects of the treatments on cell viability.
Figure 2.
NSL-YHJ-2-27 blocks proliferation and induces cell rounding and death of breast cancer cells. Cells were seeded onto six-well plates and then treated with the indicated concentrations (0, 1, 2, and 5 µM) of PCAIs for 48 h. (A) Images of the cells were then captured using Nikon Ti Eclipse microscope at 10× magnification. Scale bar = 100 µm. (B) The number of cells that proliferated after PCAI treatment was obtained by subtracting the final number of cells from the initial count of cells before PCAI treatment. Significance was calculated from three independent experiments. Statistical significance (**, p < 0.01; ***, p < 0.001; ****, p < 0.0001) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 2.
NSL-YHJ-2-27 blocks proliferation and induces cell rounding and death of breast cancer cells. Cells were seeded onto six-well plates and then treated with the indicated concentrations (0, 1, 2, and 5 µM) of PCAIs for 48 h. (A) Images of the cells were then captured using Nikon Ti Eclipse microscope at 10× magnification. Scale bar = 100 µm. (B) The number of cells that proliferated after PCAI treatment was obtained by subtracting the final number of cells from the initial count of cells before PCAI treatment. Significance was calculated from three independent experiments. Statistical significance (**, p < 0.01; ***, p < 0.001; ****, p < 0.0001) was determined by one-way ANOVA with post hoc Dunnett’s test.
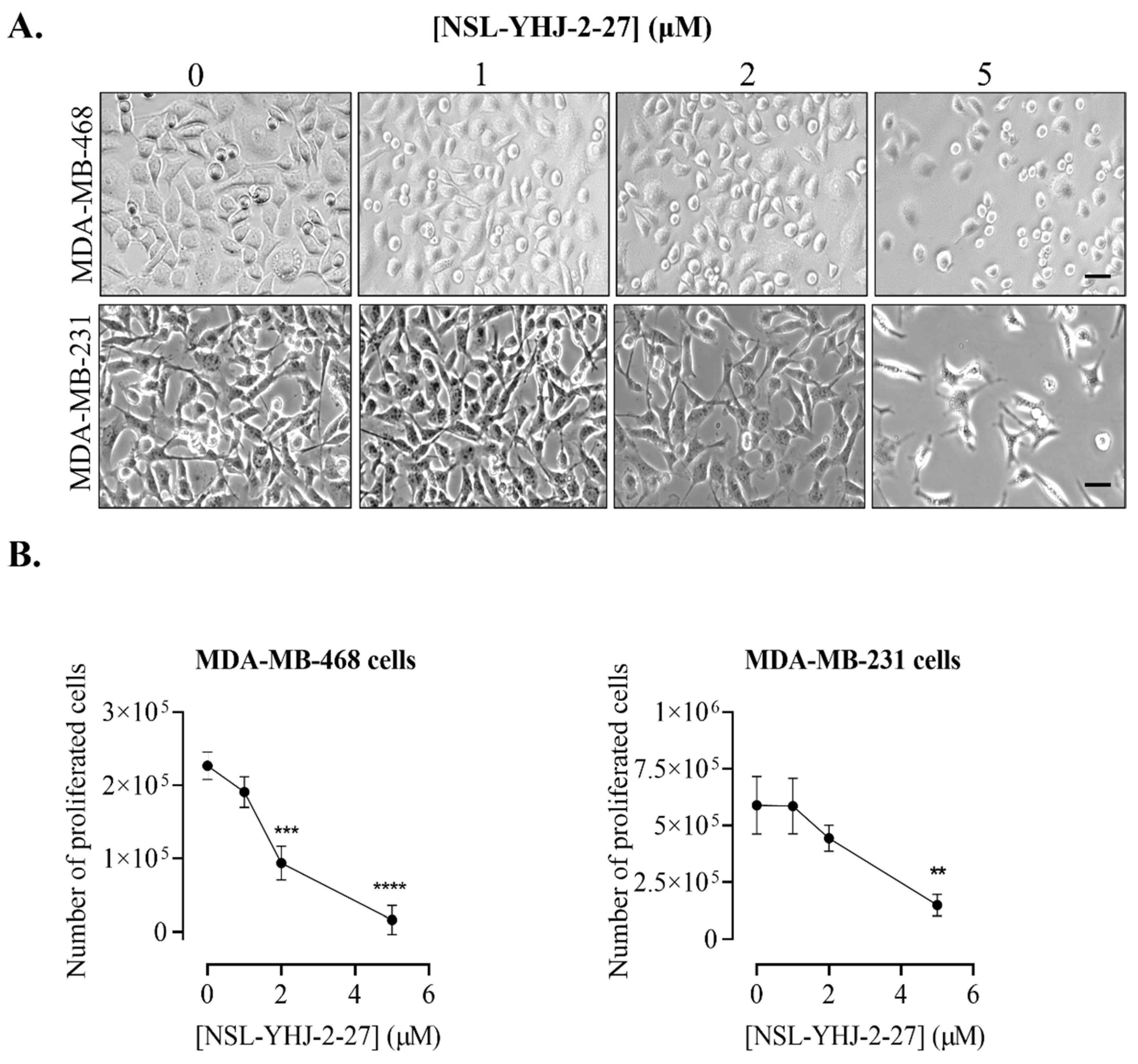
Figure 3.
Breast cancer cells do not survive long-term PCAIs treatment. Cell suspensions consisting of 100,000 cells were seeded onto twelve-well plates and exposed to the indicated concentrations of PCAIs (0, 1, 2, and 5 µM). Images were captured daily at 10× magnification using Nikon Ti Eclipse microscope. Scale bar = 100 µm.
Figure 3.
Breast cancer cells do not survive long-term PCAIs treatment. Cell suspensions consisting of 100,000 cells were seeded onto twelve-well plates and exposed to the indicated concentrations of PCAIs (0, 1, 2, and 5 µM). Images were captured daily at 10× magnification using Nikon Ti Eclipse microscope. Scale bar = 100 µm.
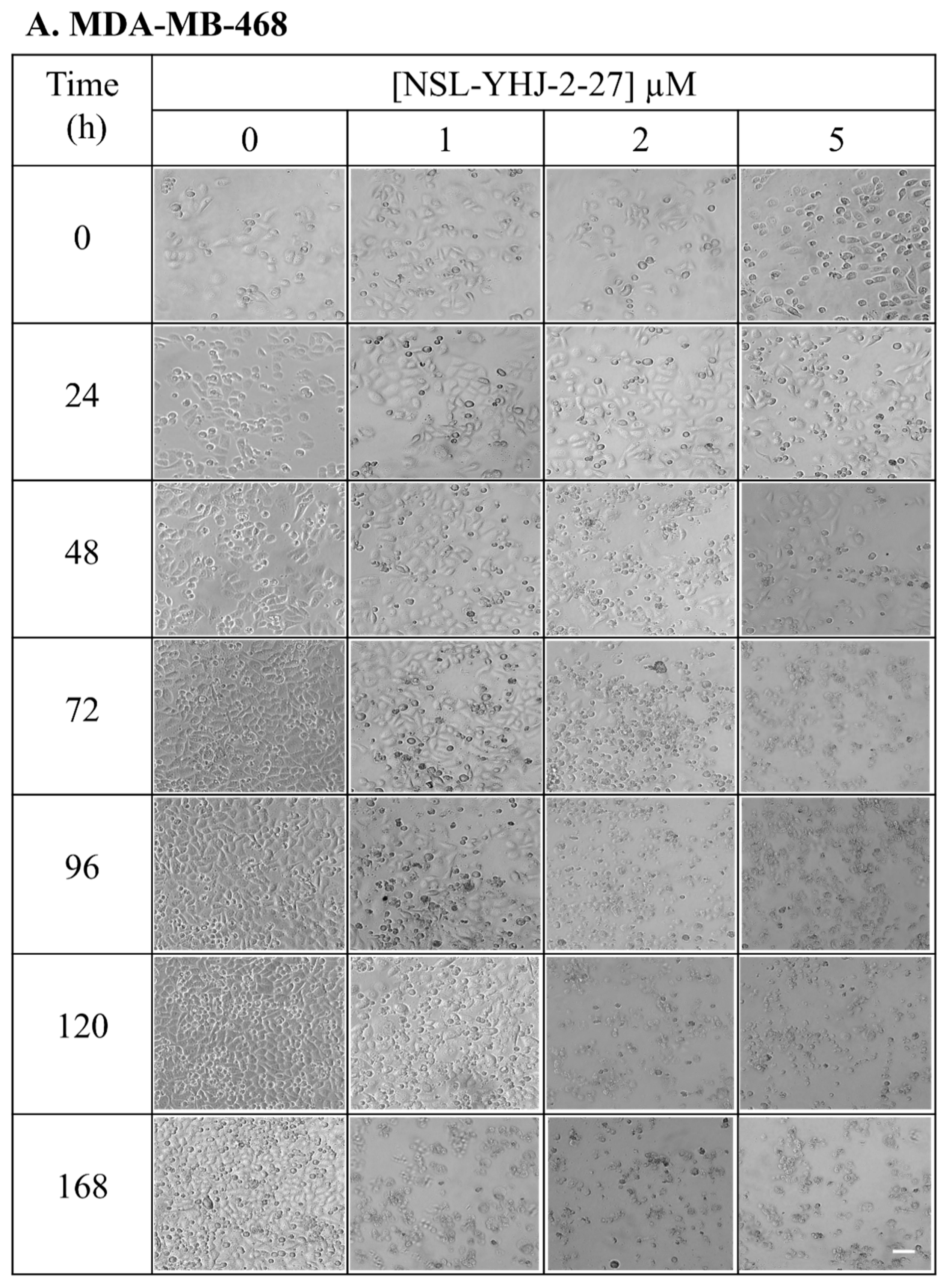
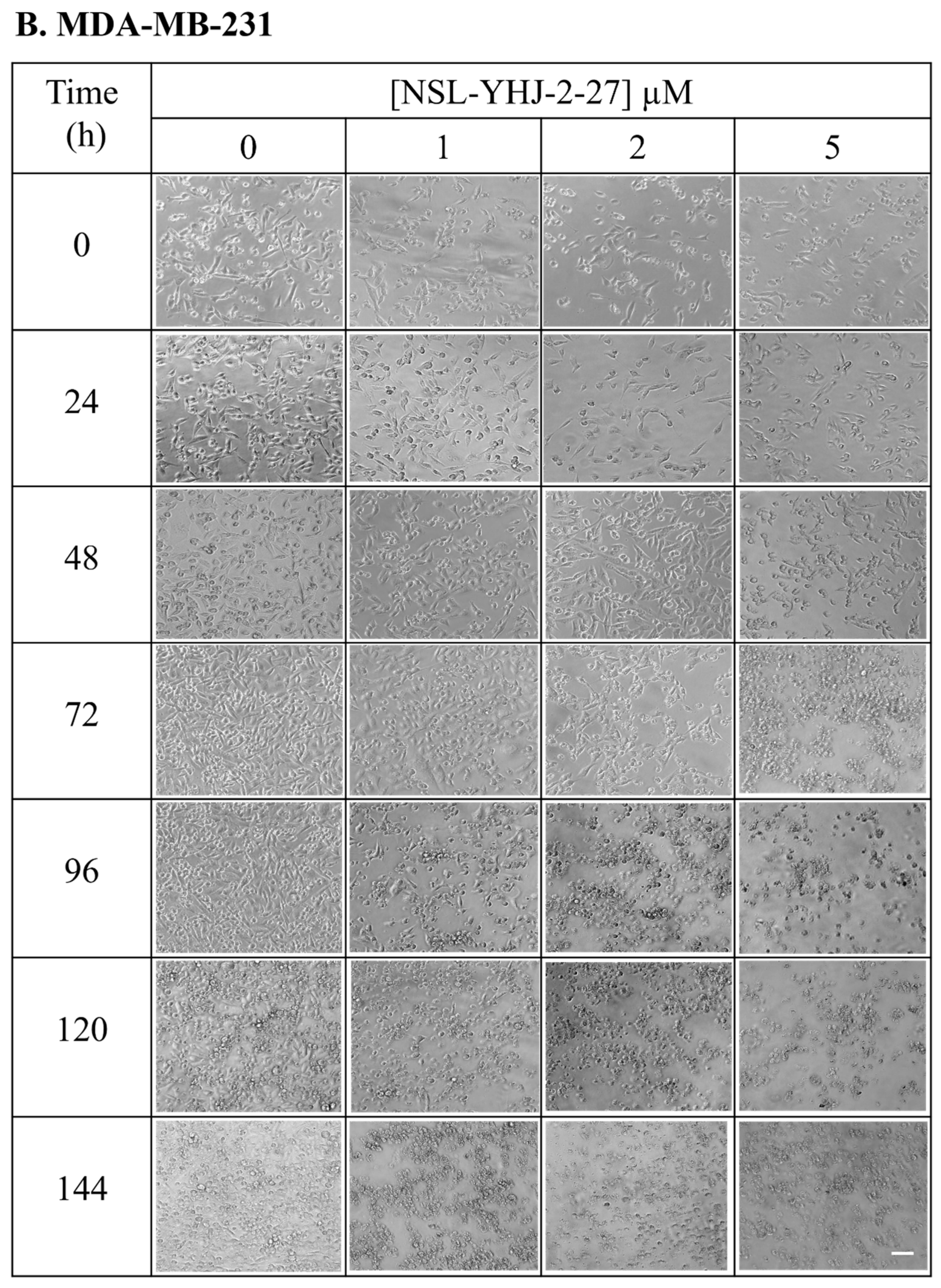
Figure 4.
NSL-YHJ-2-27 exerts pronounced effects on the phosphorylation of MAPK pathway enzymes. Cells were treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, and 5 µM) or the non-farnesylated analog NSL-YHJ-2-62 (10 µM) for 48 h. They were then lysed and analyzed by Western blotting as described in the Methods section. Data are representative of three independent experiments. Statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001; **** p < 0.0001) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 4.
NSL-YHJ-2-27 exerts pronounced effects on the phosphorylation of MAPK pathway enzymes. Cells were treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, and 5 µM) or the non-farnesylated analog NSL-YHJ-2-62 (10 µM) for 48 h. They were then lysed and analyzed by Western blotting as described in the Methods section. Data are representative of three independent experiments. Statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001; **** p < 0.0001) was determined by one-way ANOVA with post hoc Dunnett’s test.
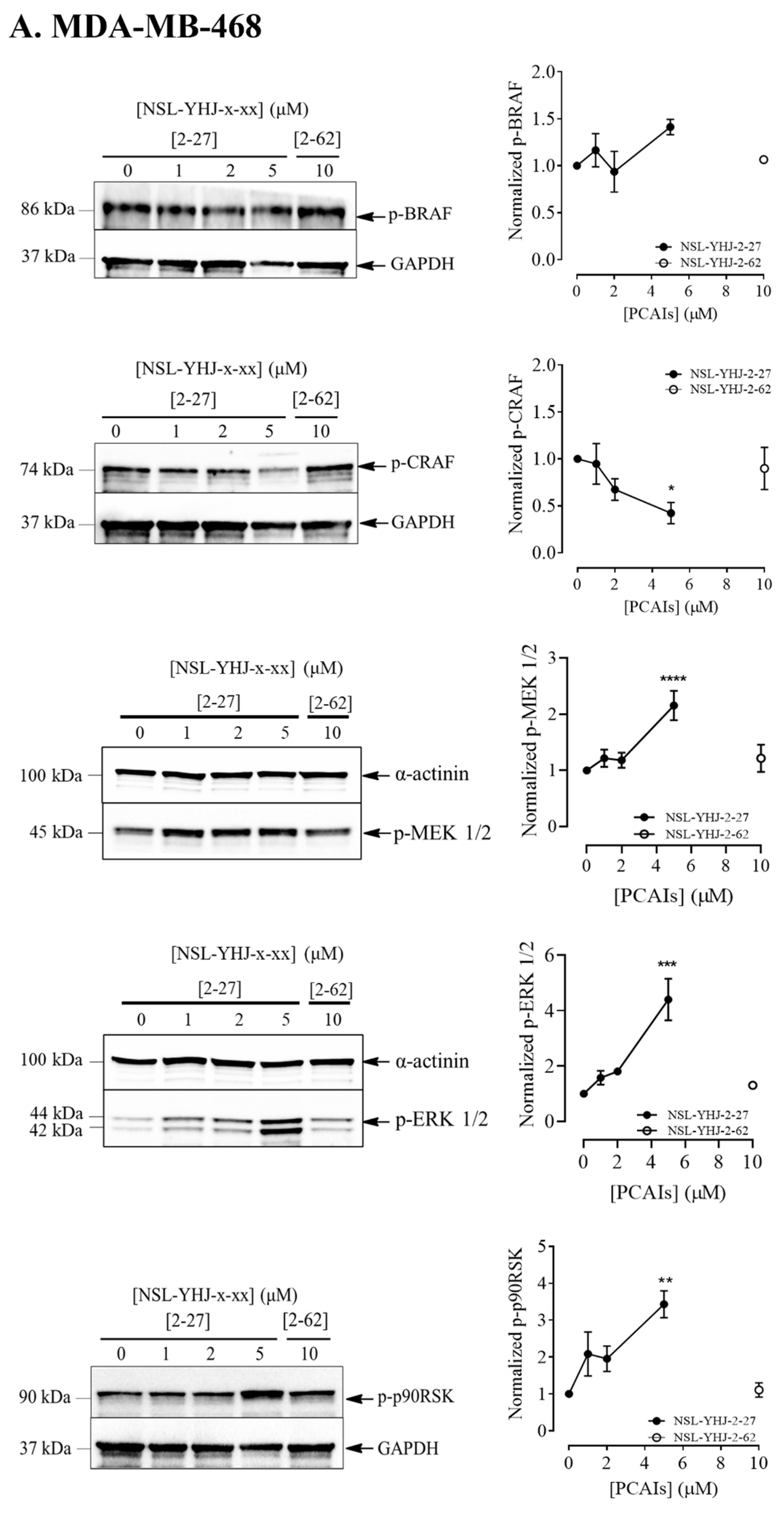
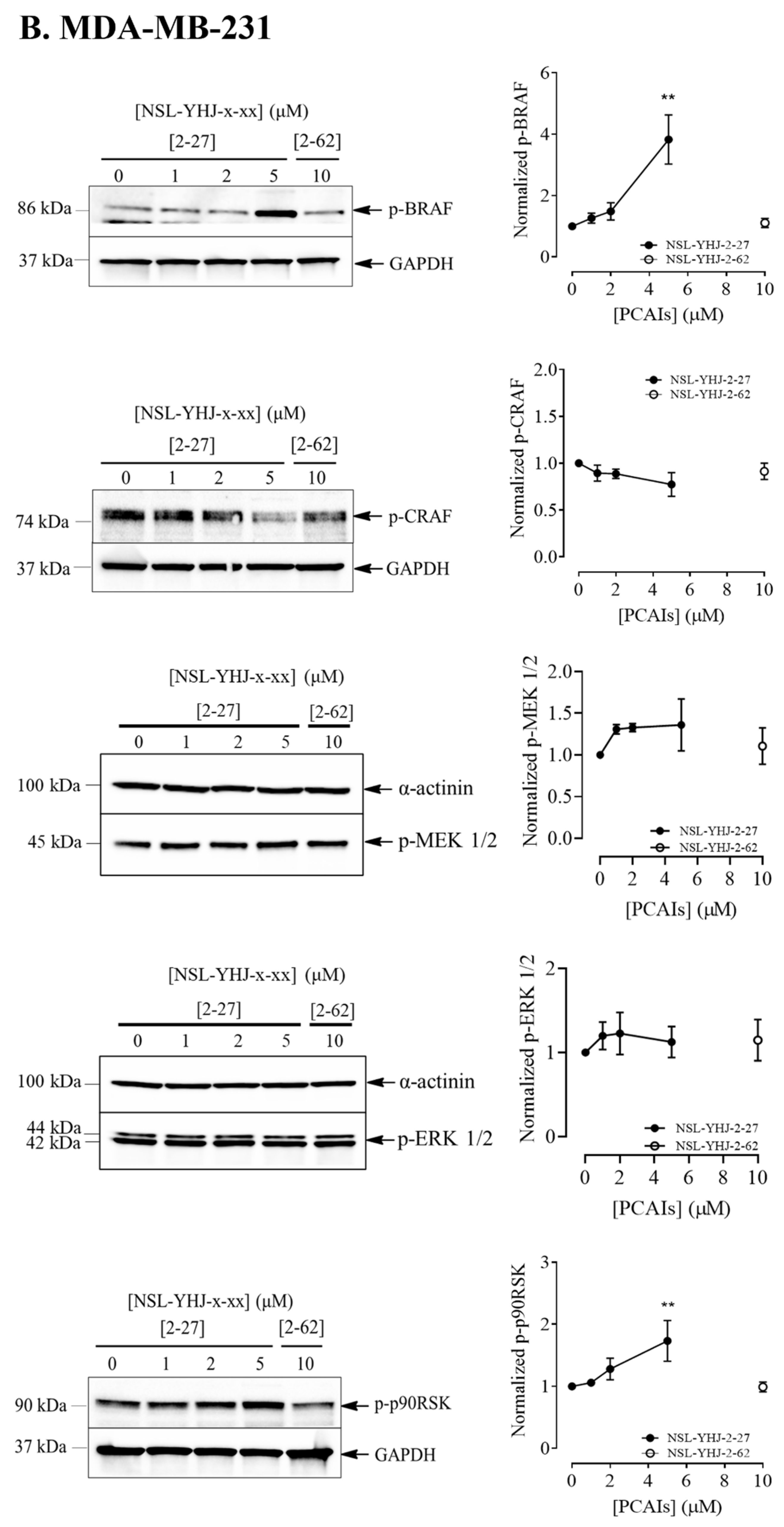
Figure 5.
NSL-YHJ-2-27 reduces AKT phosphorylation in KRAS-mutant breast cancer cells. (A,B) Cells were treated with the indicated concentrations of PCAIs NSL-YHJ-2-27 (0, 1, 2, and 5 µM) or NSL-YHJ-2-62 (10 µM) and analyzed for AKT phosphorylation levels by Western blotting, as described in the Methods. Data are representative of three independent experiments. Statistical significance (***, p < 0.001, ****, p < 0.0001) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 5.
NSL-YHJ-2-27 reduces AKT phosphorylation in KRAS-mutant breast cancer cells. (A,B) Cells were treated with the indicated concentrations of PCAIs NSL-YHJ-2-27 (0, 1, 2, and 5 µM) or NSL-YHJ-2-62 (10 µM) and analyzed for AKT phosphorylation levels by Western blotting, as described in the Methods. Data are representative of three independent experiments. Statistical significance (***, p < 0.001, ****, p < 0.0001) was determined by one-way ANOVA with post hoc Dunnett’s test.
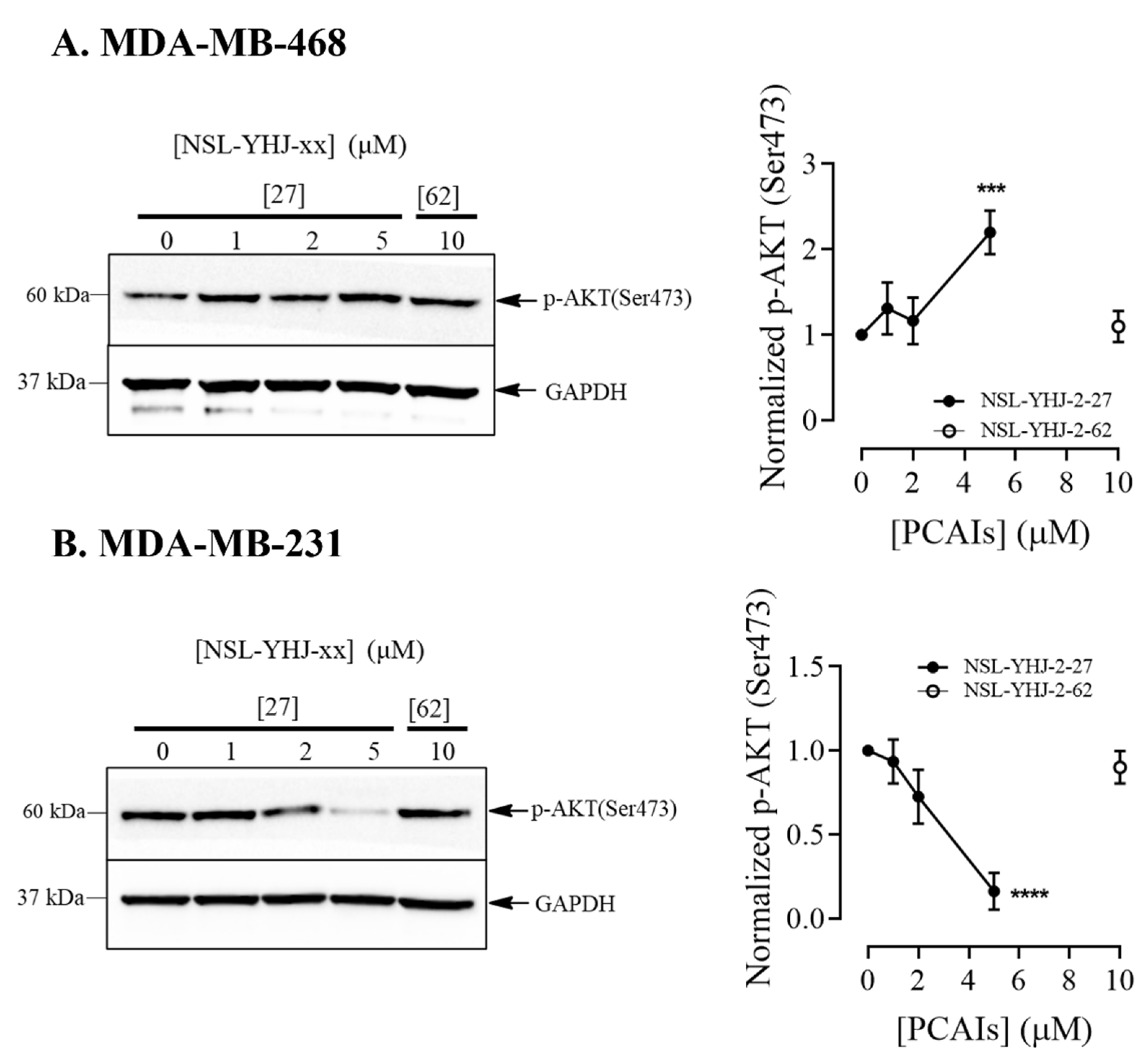
Figure 6.
NSL-YHJ-2-27 induces apoptosis in MDA-MB-468 cells. (A) Cells were treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, and 5 µM), collected, and stained with FLICA and propidium iodide, followed by flow cytometry analysis. (B) PCAIs-treated cells, NSL-YHJ-2-27 (0, 1, 2, and 5 µM) or NSL-YHJ-2-62 (10 µM), were analyzed for apoptotic factors by Western blotting, as described in the Methods. Data are representative of three independent experiments. Statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 6.
NSL-YHJ-2-27 induces apoptosis in MDA-MB-468 cells. (A) Cells were treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, and 5 µM), collected, and stained with FLICA and propidium iodide, followed by flow cytometry analysis. (B) PCAIs-treated cells, NSL-YHJ-2-27 (0, 1, 2, and 5 µM) or NSL-YHJ-2-62 (10 µM), were analyzed for apoptotic factors by Western blotting, as described in the Methods. Data are representative of three independent experiments. Statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
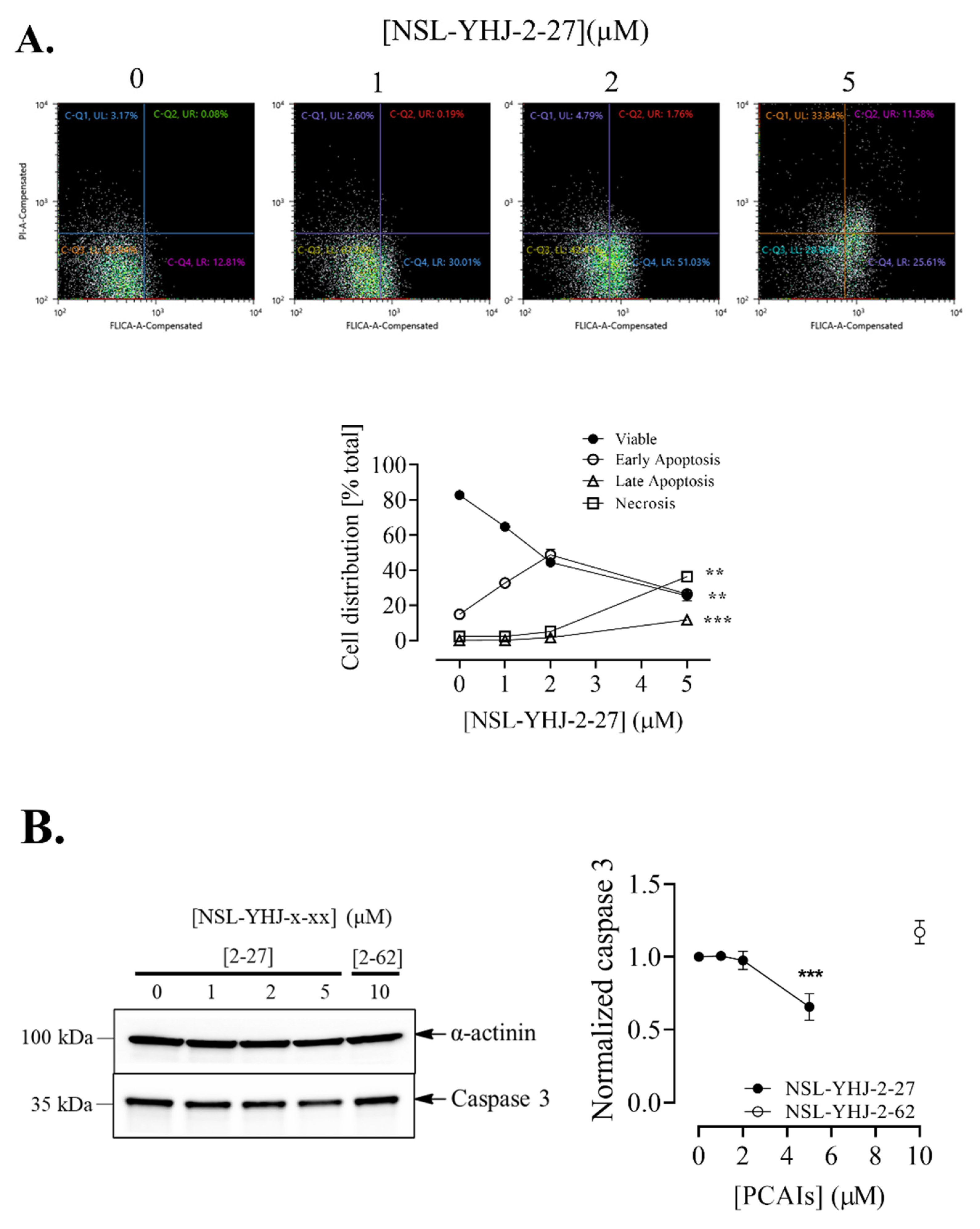
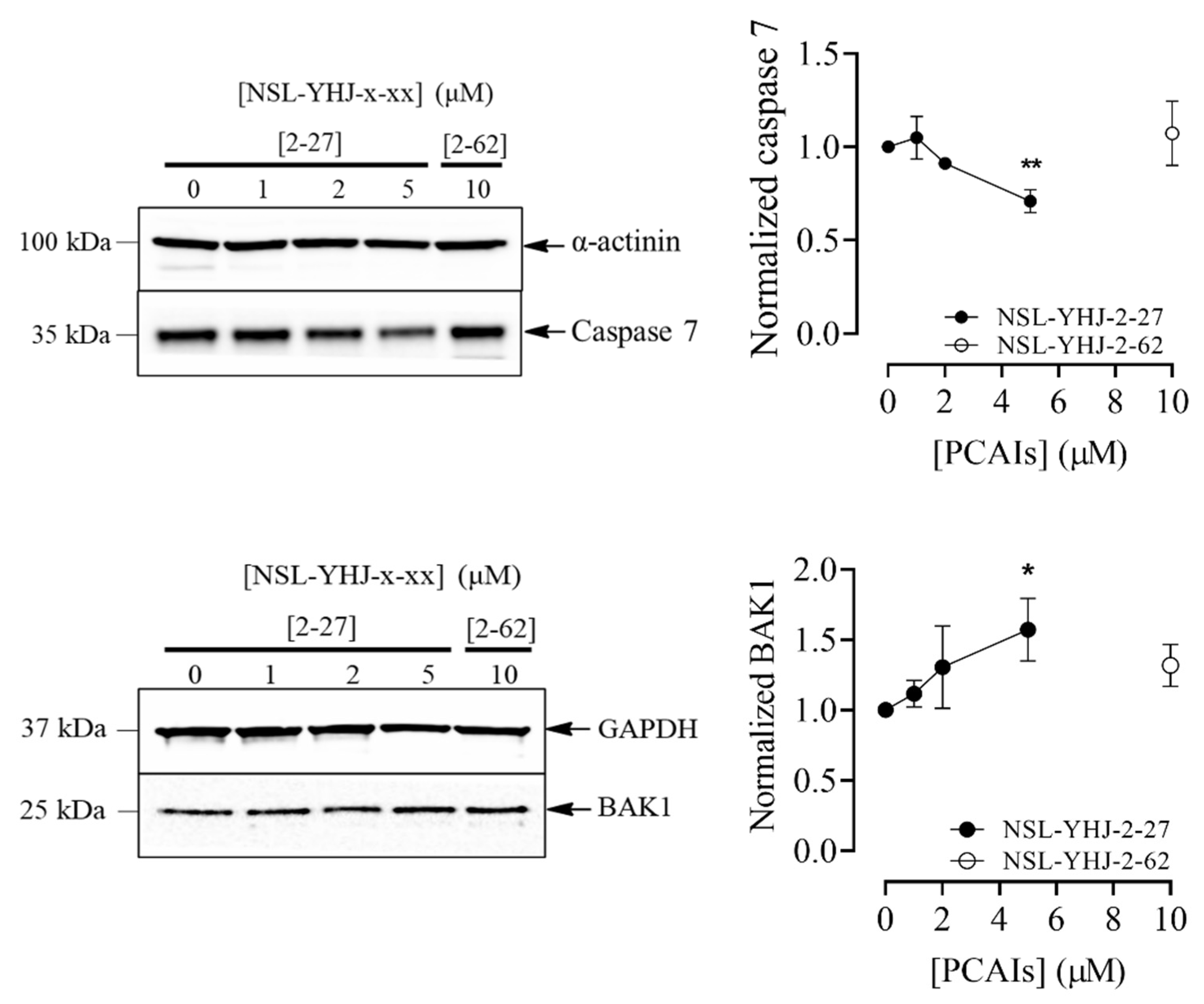
Figure 7.
NSL-YHJ-2-27 treatment disaggregates compact spheroids. MDA-MB-468 and MDA-MB-231 cells were seeded in 96U round bottom Nunclon Sphera Plate and allowed to grow for 48 h. Once compact spheroids were formed, they were treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, 5, and 10 µM) and observed for 72 h. They were then stained with 10 µg/mL of AO/EB dye mixture at 72 h post-PCAIs-treatment and brightfield (BF) (A,B) and fluorescent (C,D) images were captured at 4× magnification using a Nikon Ti Eclipse microscope. Scale bar = 100 µm. The mean intensity ratios AO/EB equivalent to the viable-to-dead cells in the spheroids were determined (E,F) from three replicates. Statistical significance (*, p < 0.05; ***, p < 0.001) was determined by 1-way ANOVA with post hoc Dunnett’s test.
Figure 7.
NSL-YHJ-2-27 treatment disaggregates compact spheroids. MDA-MB-468 and MDA-MB-231 cells were seeded in 96U round bottom Nunclon Sphera Plate and allowed to grow for 48 h. Once compact spheroids were formed, they were treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, 5, and 10 µM) and observed for 72 h. They were then stained with 10 µg/mL of AO/EB dye mixture at 72 h post-PCAIs-treatment and brightfield (BF) (A,B) and fluorescent (C,D) images were captured at 4× magnification using a Nikon Ti Eclipse microscope. Scale bar = 100 µm. The mean intensity ratios AO/EB equivalent to the viable-to-dead cells in the spheroids were determined (E,F) from three replicates. Statistical significance (*, p < 0.05; ***, p < 0.001) was determined by 1-way ANOVA with post hoc Dunnett’s test.
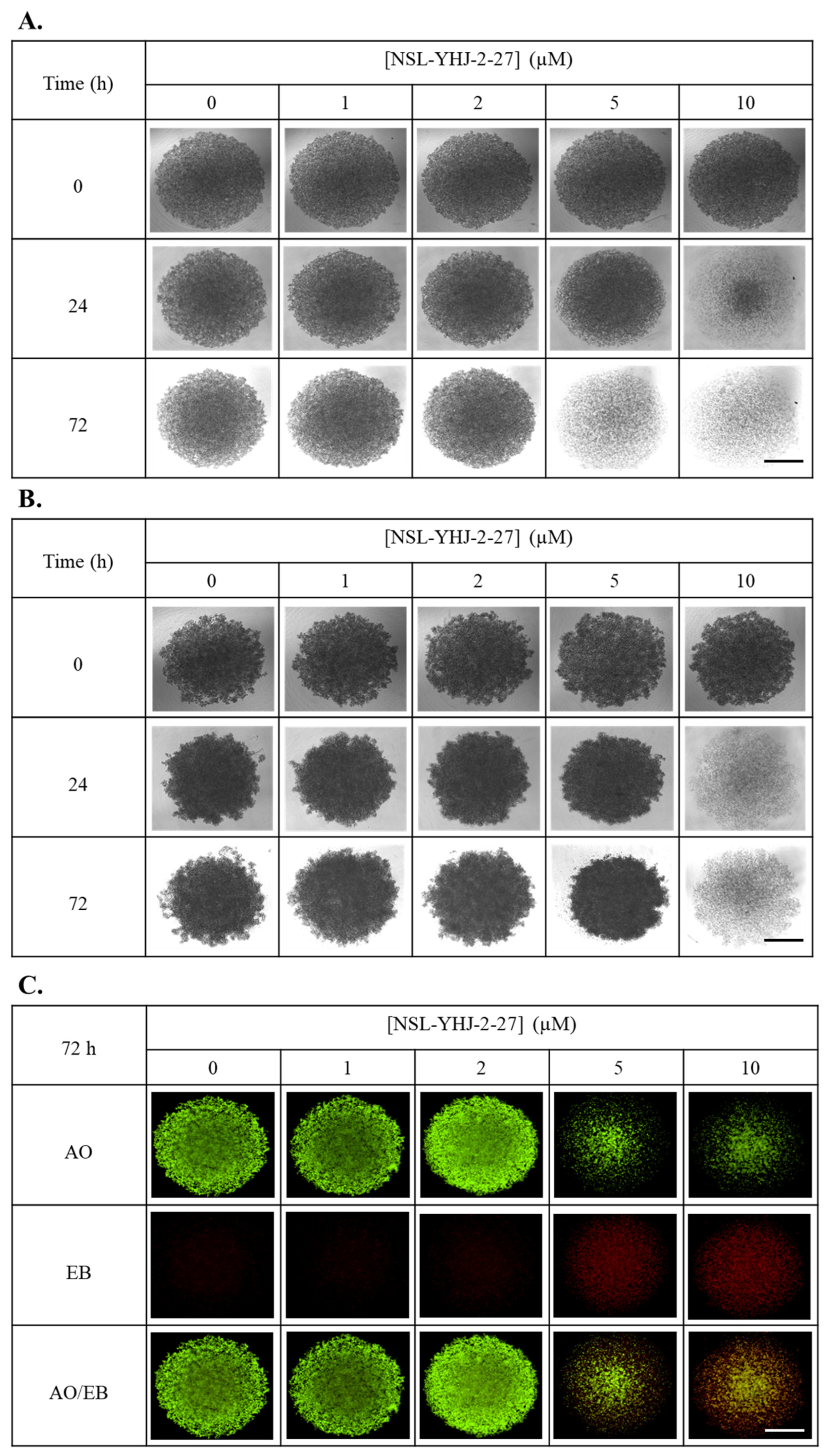
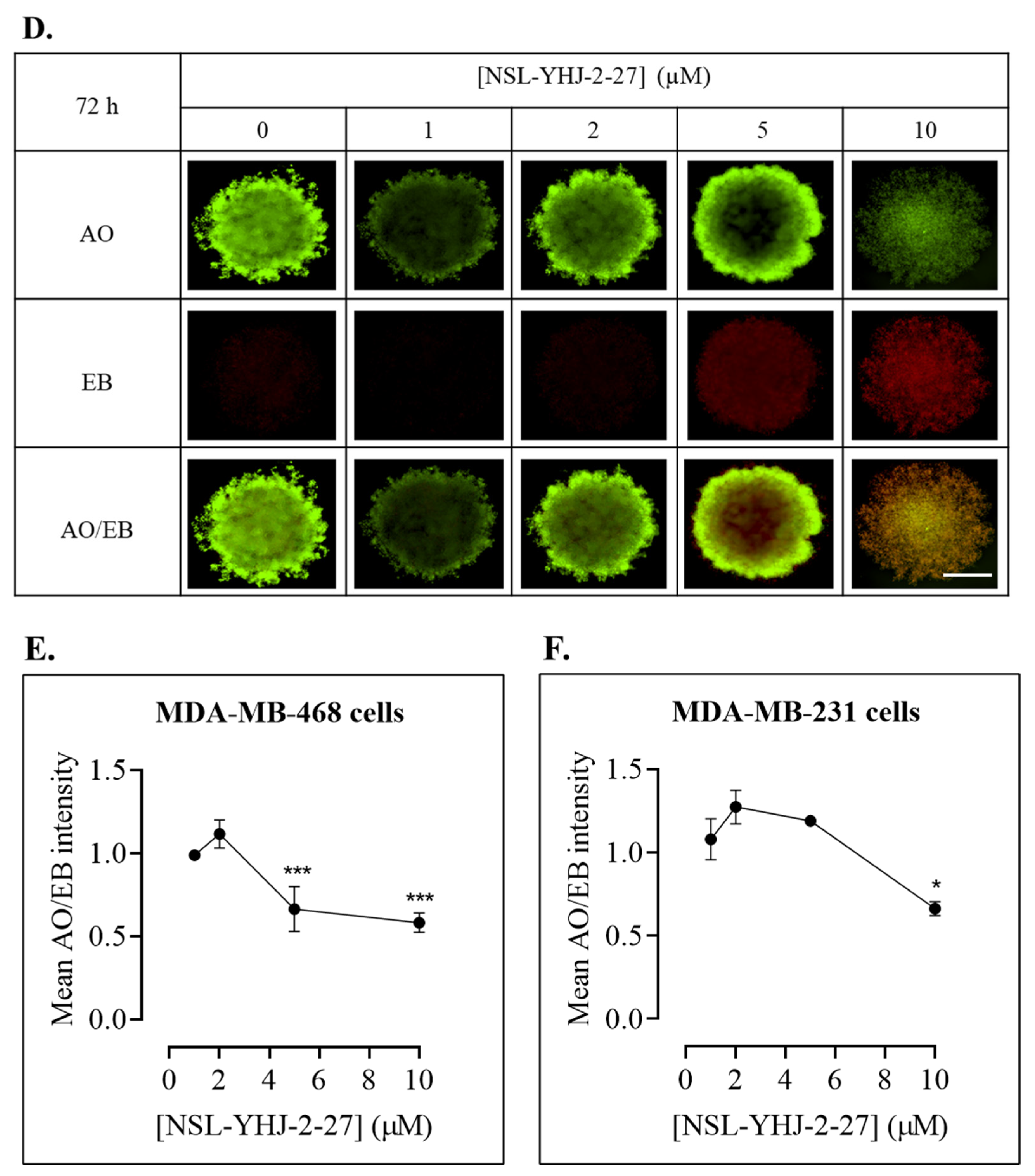
Figure 8.
RSK1/2/3 inhibition does not reverse PCAIs-induced cell death. After treatment of cells with varying concentrations of NSL-YHJ-2-27 or RSK1/2/3 inhibitor (BRD7389) and varying concentrations of NSL-YHJ-2-27 in combination with the indicated concentrations of the RSK1/2/3 inhibitor for 48 h, resazurin reduction assay was performed (A,B) and EC50 value was calculated from non-linear regression (curve fit) using GraphPad Prism (C) from four replicates.
Figure 8.
RSK1/2/3 inhibition does not reverse PCAIs-induced cell death. After treatment of cells with varying concentrations of NSL-YHJ-2-27 or RSK1/2/3 inhibitor (BRD7389) and varying concentrations of NSL-YHJ-2-27 in combination with the indicated concentrations of the RSK1/2/3 inhibitor for 48 h, resazurin reduction assay was performed (A,B) and EC50 value was calculated from non-linear regression (curve fit) using GraphPad Prism (C) from four replicates.
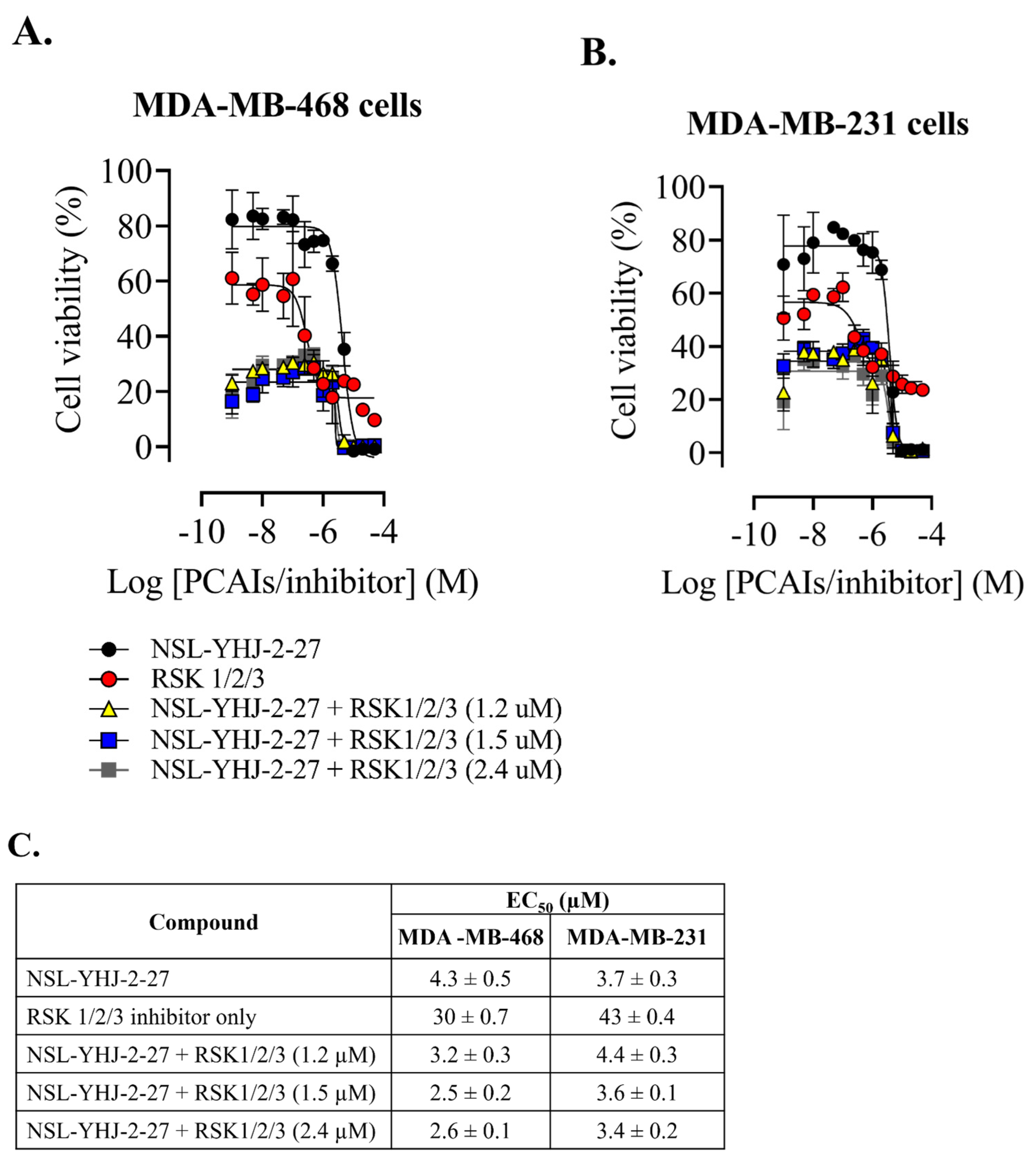
Figure 9.
NSL-YHJ-2-27 inhibits breast cancer cell invasion into Matrigel. MDA-MB-231 cells were seeded in 96U round bottom Nunclon Sphera plates and allowed to grow for 48 h. The preformed spheroids were then treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, and 5 µM), after which they were embedded in a Matrigel containing the corresponding concentrations of NSL-YHJ-2-27 in three replicates. Once the Matrigel solidified, brightfield (BF) images were obtained using a Nikon Ti Eclipse microscope at 4× magnification for 0 h exposure. (A) The spheroids were incubated for another 72 h, after which BF images were again obtained. Scale bar = 100 µm. (B) The area invaded by the cells was measured from the body of the spheroid using the NIS Element software. Statistical significance (***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 9.
NSL-YHJ-2-27 inhibits breast cancer cell invasion into Matrigel. MDA-MB-231 cells were seeded in 96U round bottom Nunclon Sphera plates and allowed to grow for 48 h. The preformed spheroids were then treated with the indicated concentrations of NSL-YHJ-2-27 (0, 1, 2, and 5 µM), after which they were embedded in a Matrigel containing the corresponding concentrations of NSL-YHJ-2-27 in three replicates. Once the Matrigel solidified, brightfield (BF) images were obtained using a Nikon Ti Eclipse microscope at 4× magnification for 0 h exposure. (A) The spheroids were incubated for another 72 h, after which BF images were again obtained. Scale bar = 100 µm. (B) The area invaded by the cells was measured from the body of the spheroid using the NIS Element software. Statistical significance (***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
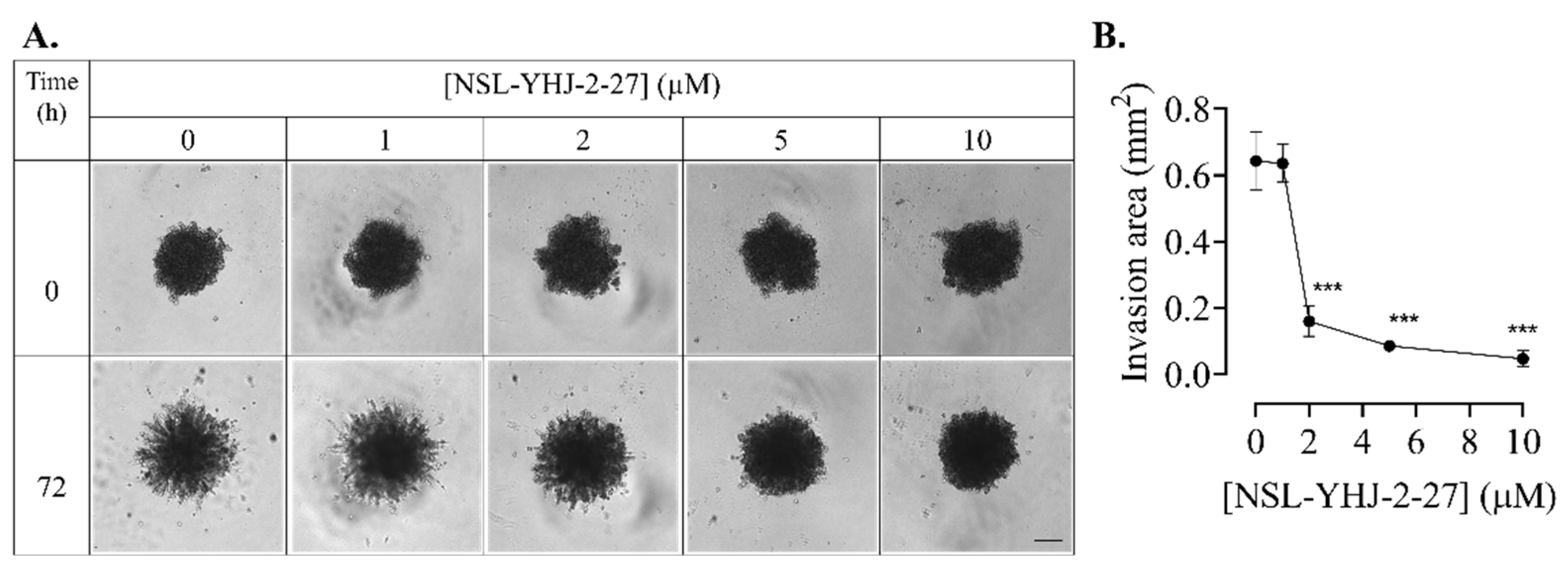
Figure 10.
NSL-YHJ-2-27 disrupts F-actin filaments. Cells were grown in 8-well ibidi µ-slide until adherence. They were then treated with PCAIs for 48 h, followed by fixing and permeabilization. Actin filaments and nuclei were stained by incubating the cells in Alexa Fluor 568 Phalloidin and Hoechst, respectively. (A) Images were captured at 40× magnification using Keyence fluorescence microscope. Scale bar = 100 µm. (B) Total cellular fluorescence intensity was obtained by quantifying and averaging the intensities for n = 300 cells using Keyence BZ-X800 analyzer. Statistical significance (***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 10.
NSL-YHJ-2-27 disrupts F-actin filaments. Cells were grown in 8-well ibidi µ-slide until adherence. They were then treated with PCAIs for 48 h, followed by fixing and permeabilization. Actin filaments and nuclei were stained by incubating the cells in Alexa Fluor 568 Phalloidin and Hoechst, respectively. (A) Images were captured at 40× magnification using Keyence fluorescence microscope. Scale bar = 100 µm. (B) Total cellular fluorescence intensity was obtained by quantifying and averaging the intensities for n = 300 cells using Keyence BZ-X800 analyzer. Statistical significance (***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
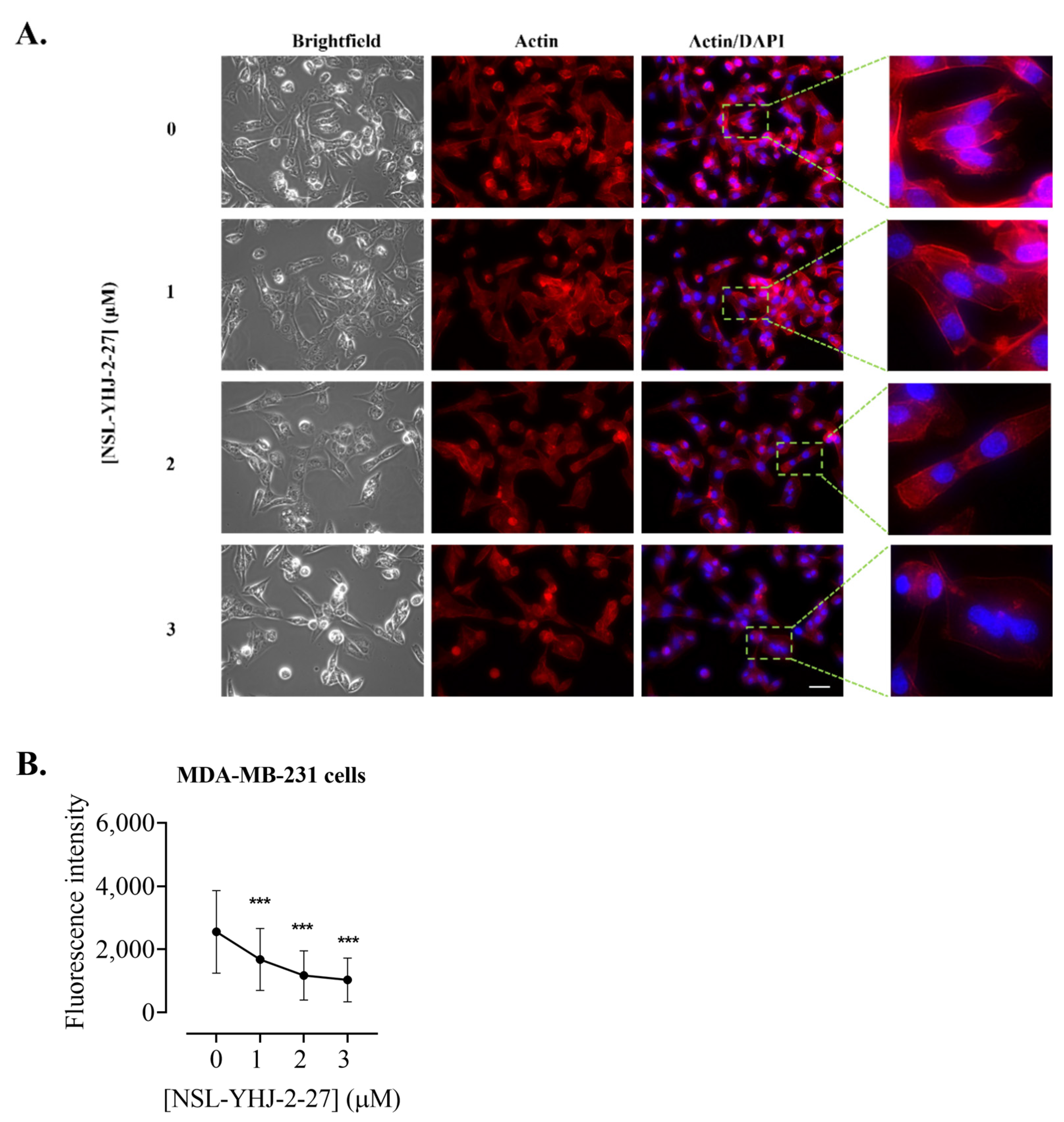
Figure 11.
NSL-YHJ-2-27 disrupts the assembly of vinculin, leading to cell shrinkage. Cells were grown in 8-well ibidi µ-slides until adherence. They were then treated with PCAIs for 48 h and analyzed by immunocytochemistry for vinculin localization using anti-rabbit IgG Alexa Fluor 555 conjugate, as described in the Methods. Images were captured using Keyence fluorescence microscope at 40× magnification. Scale bar = 100 µm. (A). White arrows indicate the original ‘footprints’ of dislodged cells. In (B,C), n = 60 to 100 cells were quantified for fluorescence intensity and area using Keyence BZ-X800 analyzer. Data were plotted using GraphPad Prism version 8. Statistical significance (***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 11.
NSL-YHJ-2-27 disrupts the assembly of vinculin, leading to cell shrinkage. Cells were grown in 8-well ibidi µ-slides until adherence. They were then treated with PCAIs for 48 h and analyzed by immunocytochemistry for vinculin localization using anti-rabbit IgG Alexa Fluor 555 conjugate, as described in the Methods. Images were captured using Keyence fluorescence microscope at 40× magnification. Scale bar = 100 µm. (A). White arrows indicate the original ‘footprints’ of dislodged cells. In (B,C), n = 60 to 100 cells were quantified for fluorescence intensity and area using Keyence BZ-X800 analyzer. Data were plotted using GraphPad Prism version 8. Statistical significance (***, p < 0.001) was determined by one-way ANOVA with post hoc Dunnett’s test.
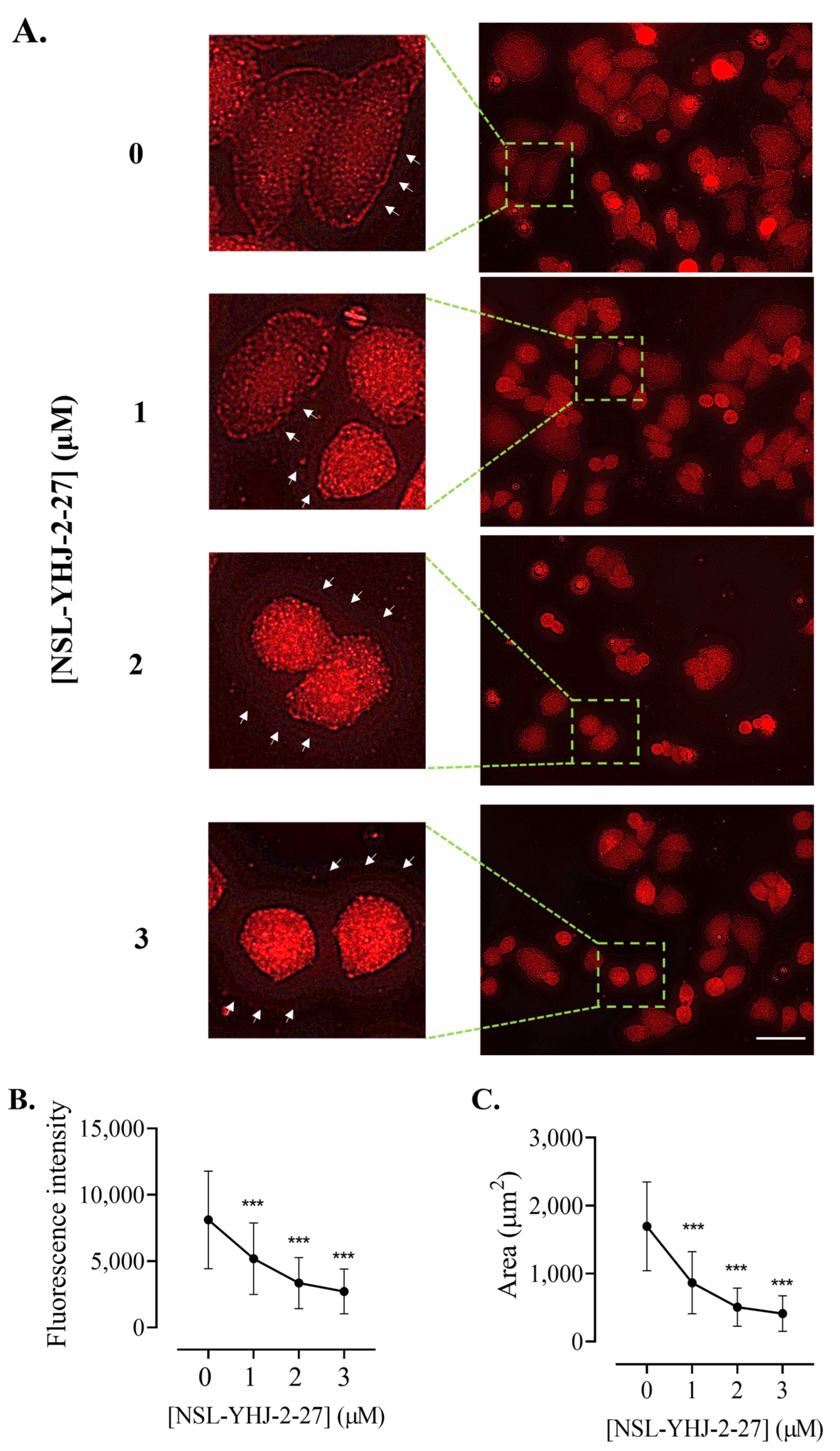
Figure 12.
NSL-YHU-2-27 decreases the level of vinculin in MDA-MB-231 cells. Cells were treated for 48 h with the indicated concentrations of NSL-YHJ-2-27 or 10 μM of the non-farnesylated PCAIs analog NSL-YHJ-2-62. They were then lysed and analyzed by Western blotting for vinculin, as described in the Methods. (A) Western blot images and (B) chemiluminescence plots of bands following quantification using Image Lab software, normalized against GAPDH. Data are representative of three independent experiments. Statistical significance (*, p < 0.05) was determined by one-way ANOVA with post hoc Dunnett’s test.
Figure 12.
NSL-YHU-2-27 decreases the level of vinculin in MDA-MB-231 cells. Cells were treated for 48 h with the indicated concentrations of NSL-YHJ-2-27 or 10 μM of the non-farnesylated PCAIs analog NSL-YHJ-2-62. They were then lysed and analyzed by Western blotting for vinculin, as described in the Methods. (A) Western blot images and (B) chemiluminescence plots of bands following quantification using Image Lab software, normalized against GAPDH. Data are representative of three independent experiments. Statistical significance (*, p < 0.05) was determined by one-way ANOVA with post hoc Dunnett’s test.
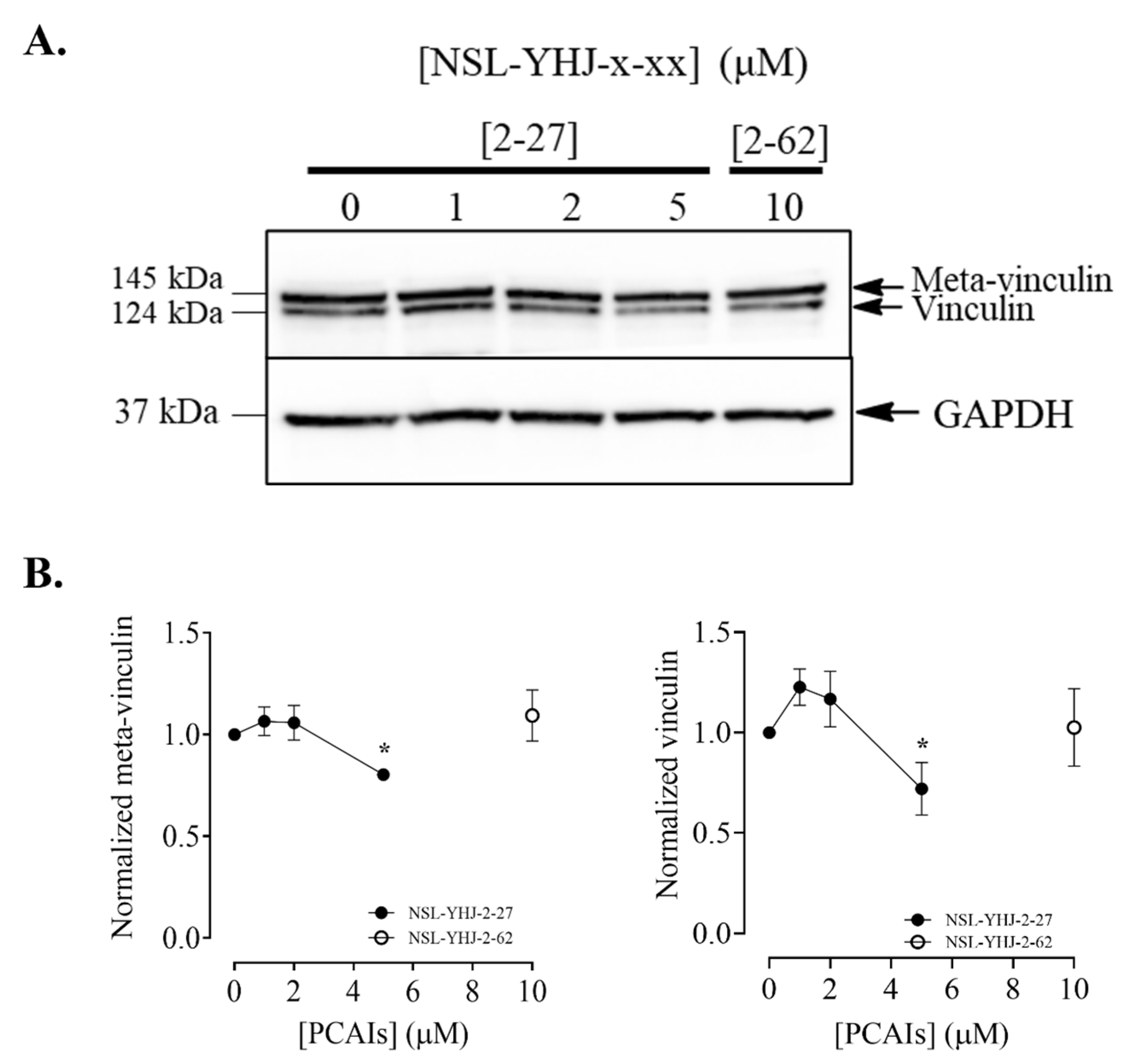
Figure 13.
Proposed mechanism of action of the PCAIs in breast cancer cell lines. Abbreviations: RTK, Receptor Tyrosine Kinase; GRB2, Growth Factor Receptor Bound Protein 2; SOS, Son-of-Sevenless; GDP, Guanosine Diphosphate; GTP, Guanosine Triphosphate; RAS, Rat Sarcoma; BRAF, Rapidly Accelerated Fibrosarcoma, v-Raf Murine Sarcoma Viral Oncogene Homolog B; CRAF, RAF Proto-Oncogene Serine/Threonine-Protein Kinase; MEK, Mitogen-Activated Protein Kinase Kinase; ERK, Extracellular-Signal-Regulated Kinases; p90RSK, 90 kDa Ribosomal s6 Kinases; AKT, Protein Kinase B; BAK1, BCL2 Antagonist/Killer 1.
Figure 13.
Proposed mechanism of action of the PCAIs in breast cancer cell lines. Abbreviations: RTK, Receptor Tyrosine Kinase; GRB2, Growth Factor Receptor Bound Protein 2; SOS, Son-of-Sevenless; GDP, Guanosine Diphosphate; GTP, Guanosine Triphosphate; RAS, Rat Sarcoma; BRAF, Rapidly Accelerated Fibrosarcoma, v-Raf Murine Sarcoma Viral Oncogene Homolog B; CRAF, RAF Proto-Oncogene Serine/Threonine-Protein Kinase; MEK, Mitogen-Activated Protein Kinase Kinase; ERK, Extracellular-Signal-Regulated Kinases; p90RSK, 90 kDa Ribosomal s6 Kinases; AKT, Protein Kinase B; BAK1, BCL2 Antagonist/Killer 1.
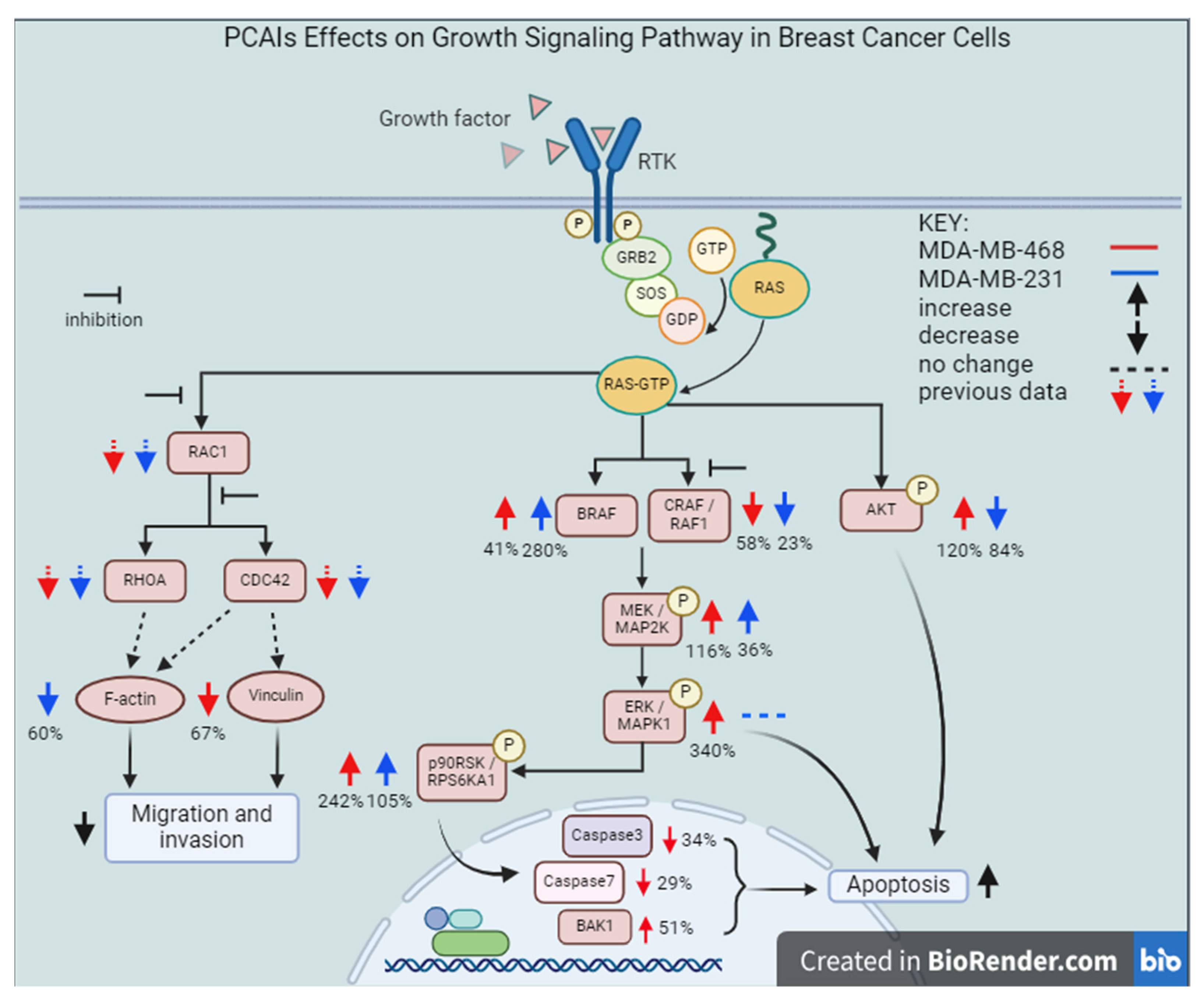
Table 1.
PCAIs structure. The structure of the experimental PCAIs, NSL-YHJ-2-27, and control PCAIs analog without the polyisoprenyl moiety NSL-YHJ-2-62.
Table 1.
PCAIs structure. The structure of the experimental PCAIs, NSL-YHJ-2-27, and control PCAIs analog without the polyisoprenyl moiety NSL-YHJ-2-62.
| Compound | Structure |
|---|---|
| NSL-YHJ-2-27 |  |
| NSL-YHJ-2-62 |  |
[ad_2]