JCM | Free Full-Text | Anti-Arrhythmic Effects of Heart Failure Guideline-Directed Medical Therapy and Their Role in the Prevention of Sudden Cardiac Death: From Beta-Blockers to Sodium-Glucose Cotransporter 2 Inhibitors and Beyond
[ad_1]
1. Introduction
2. Pathophysiology of Ventricular Arrhythmia and Sudden Cardiac Death
3. Beta-Blockers
In conclusion, BB substantially reduce proarrhythmic risk by inhibiting sympathetically mediated triggers, reducing functional reentrant substrates, and slowing the sinus node and atrioventricular nodal rates. The evidence supporting their use in HFrEF is robust and emphasizes their critical role in mitigating arrhythmias and SCD in this patient population.
4. Angiotensin-Converting Enzyme Inhibitor/Angiotensin Receptor Blocker
In conclusion, although ACEi and ARB have demonstrated favorable effects on the RAAS and have robust data supporting their beneficial impact on overall outcomes in HFrEF, their role in reducing the risk of SCD has not been definitively established, as underscored by multiple meta-analyses.
5. Angiotensin Receptor–Neprilysin Inhibitor
In conclusion, ARNi therapy appears to manifest favorable effects via multiple mechanisms, including vasodilation, the attenuation of sympathetic activation, the reduction in myocardial wall stretch and fibrosis, and modulatory impacts on ion channels such as potassium channels, RyR2, and the CaMKII pathway. Meta-analyses confirm ARNi’s efficacy in reducing both VA and SCD, yet further investigation is needed to fully understand the precise molecular mechanisms.
6. Mineralocorticoid Receptor Antagonists
7. Sodium-Glucose Cotransporter 2 Inhibitors
In conclusion, SGLT2i appear to exhibit anti-arrhythmic properties through various pleiotropic mechanisms, including the restoration of calcium and sodium homeostasis, the reversal of cardiac remodeling, and the exertion of antioxidant and anti-inflammatory effects. Although current data trends suggest a reduced incidence of SCD and VA, further well-designed prospective studies are imperative for the definitive validation of these anti-arrhythmic effects.
8. Implantable Cardioverter–Defibrillator in Light of New Heart Failure Treatment
All these considerations question the exact place of ICD in this contemporary era of HF medication. In the absence of appropriate trials that incorporate the latest GDMT, adherence to existing guidelines is obligatory. However, there is a compelling need for updated research to more precisely define the indications and optimal timing for ICD implantation in the current therapeutic landscape.
9. Emerging Horizons in Heart Failure Treatment
10. Conclusions
In conclusion, SCD constitutes a critical contributor to mortality among patients with HFrEF, predominantly due to malignant VA. The optimization of GDMT serves as an effective strategy to mitigating the incidence of SCD within this population. Therapeutic interventions primarily target key systems such as the RAAS and the sympathetic nervous system, preventing both structural and electrical remodeling of the myocardium.
Despite their favorable modulatory effects on RAAS, ACEi and ARB have not been conclusively associated with a reduction in either SCD or VA. Robust evidence supports the utility of BB, ARNi, and MRA in reducing the risk of SCD. Furthermore, SGLT2i offer promising preliminary data, necessitating further well-designed prospective studies for confirmation of their anti-arrhythmic effects. Through the employment of different complementary mechanisms of action, those molecules act in synergy, highlighting the importance of optimizing GDMT to reduce arrhythmic risk in HFrEF.
Overall, the landscape of pharmacological interventions for HFrEF is progressively expanding, with novel agents undergoing evaluation. Further research is imperative for the unequivocal delineation of these agents’ impact on SCD and VA, thus augmenting our arsenal in the management of HFrEF.
Author Contributions
Conceptualization, W.Z. and A.S.; literature research, W.Z.; writing—original draft, W.Z.; writing—review and editing, L.P., D.G.D.R. and A.S.; supervision and resources, S.B., C.d.A., G.-B.C. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kannel, W.B.; Plehn, J.F.; Cupples, L. Cardiac failure and sudden death in the Framingham Study. Am. Heart J. 1988, 115, 869–875. [Google Scholar] [CrossRef]
- Zipes, D.P.; Wellens, H.J.J. Sudden Cardiac Death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef]
- Deo, R.; Albert, C.M. Epidemiology and Genetics of Sudden Cardiac Death. Circulation 2012, 125, 620–637. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Tromp, J.; Ouwerkerk, W.; van Veldhuisen, D.J.; Hillege, H.L.; Richards, A.M.; van der Meer, P.; Anand, I.S.; Lam, C.S.; Voors, A.A. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2022, 10, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jhund, P.S.; Petrie, M.C.; Claggett, B.L.; Barlera, S.; Cleland, J.G.F.; Dargie, H.J.; Granger, C.B.; Kjekshus, J.; Køber, L.; et al. Declining Risk of Sudden Death in Heart Failure. N. Engl. J. Med. 2017, 377, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Cherry, E.M.; Fenton, F.H.; Gilmour, R.F. Mechanisms of ventricular arrhythmias: A dynamical systems-based perspective. Am. J. Physiol. Circ. Physiol. 2012, 302, H2451–H2463. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abraham, W.T. Hormones and Hemodynamics in Heart Failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Verheule, S.; Schotten, U. Electrophysiological Consequences of Cardiac Fibrosis. Cells 2021, 10, 3220. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991, 83, 1849–1865. [Google Scholar] [CrossRef]
- Mathieu, S.; El Khoury, N.; Rivard, K.; Gélinas, R.; Goyette, P.; Paradis, P.; Nemer, M.; Fiset, C. Reduction in Na+ current by angiotensin II is mediated by PKCα in mouse and human-induced pluripotent stem cell–derived cardiomyocytes. Heart Rhythm. 2016, 13, 1346–1354. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Takimoto, K.; Stewart, A.F.R.; Zhu, C.; Levitan, E.S. Independent Regulation of Cardiac Kv4.3 Potassium Channel Expression by Angiotensin II and Phenylephrine. Circ. Res. 2001, 88, 476–482. [Google Scholar] [CrossRef]
- Zhao, Z.; Fefelova, N.; Shanmugam, M.; Bishara, P.; Babu, G.J.; Xie, L.-H. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J. Mol. Cell. Cardiol. 2011, 50, 128–136. [Google Scholar] [CrossRef]
- Sag, C.M.; Wadsack, D.P.; Khabbazzadeh, S.; Abesser, M.; Grefe, C.; Neumann, K.; Opiela, M.-K.; Backs, J.; Olson, E.N.; Brown, J.H.; et al. Calcium/Calmodulin-Dependent Protein Kinase II Contributes to Cardiac Arrhythmogenesis in Heart Failure. Circ. Heart Fail. 2009, 2, 664–675. [Google Scholar] [CrossRef]
- Shannon, T.R.; Lew, W.Y. Diastolic Release of Calcium from the Sarcoplasmic Reticulum: A Potential Target for Treating Triggered Arrhythmias and Heart Failure. J. Am. Coll. Cardiol. 2009, 53, 2006–2008. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium Cycling and Signaling in Cardiac Myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Grandi, E.; Ripplinger, C.M. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol. Res. 2019, 146, 104274. [Google Scholar] [CrossRef] [PubMed]
- Barrese, V.; Taglialatela, M. New advances in beta-blocker therapy in heart failure. Front. Physiol. 2013, 4, 323. [Google Scholar] [CrossRef] [PubMed]
- CIBIS-II Investigators and committees The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 1999, 353, 9–13. [CrossRef]
- Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar] [CrossRef]
- Flather, M.D.; Shibata, M.C.; Coats, A.J.; Van Veldhuisen, D.J.; Parkhomenko, A.; Borbola, J.; Cohen-Solal, A.; Dumitrascu, D.; Ferrari, R.; Lechat, P.; et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 2005, 26, 215–225. [Google Scholar] [CrossRef]
- Packer, M.; Coats, A.J.; Fowler, M.B.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Castaigne, A.; Roecker, E.B.; et al. Effect of Carvedilol on Survival in Severe Chronic Heart Failure. N. Engl. J. Med. 2001, 344, 1651–1658. [Google Scholar] [CrossRef]
- Connolly, S.J.; Dorian, P.; Roberts, R.S.; Gent, M.; Bailin, S.; Fain, E.S.; Thorpe, K.; Champagne, J.; Talajic, M.; Coutu, B.; et al. Comparison of β-Blockers, Amiodarone Plus β-Blockers, or Sotalol for Prevention of Shocks from Implantable Cardioverter Defibrillators: The OPTIC Study: A Randomized Trial. JAMA 2006, 295, 165–171. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biondi-Zoccai, G.; Abbate, A.; D’ascenzo, F.; Castagno, D.; Van Tassell, B.; Mukherjee, D.; Lichstein, E. Benefits of blockers in patients with heart failure and reduced ejection fraction: Network meta-analysis. BMJ 2013, 346, f55. [Google Scholar] [CrossRef]
- Al-Gobari, M.; El Khatib, C.; Pillon, F.; Gueyffier, F. Beta-blockers for the prevention of sudden cardiac death in heart failure patients: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2013, 13, 52. [Google Scholar] [CrossRef]
- Al-Gobari, M.; Al-Aqeel, S.; Gueyffier, F.; Burnand, B. Effectiveness of drug interventions to prevent sudden cardiac death in patients with heart failure and reduced ejection fraction: An overview of systematic reviews. BMJ Open 2018, 8, e021108. [Google Scholar] [CrossRef]
- Chatzidou, S.; Kontogiannis, C.; Tsilimigras, D.I.; Georgiopoulos, G.; Kosmopoulos, M.; Papadopoulou, E.; Vasilopoulos, G.; Rokas, S. Propranolol Versus Metoprolol for Treatment of Electrical Storm in Patients with Implantable Cardioverter-Defibrillator. J. Am. Coll. Cardiol. 2018, 71, 1897–1906. [Google Scholar] [CrossRef]
- Diamond, A.; Goldenberg, I.; Younis, A.; Goldenberg, I.; Sampath, R.; Kutyifa, V.; Chen, A.Y.; McNitt, S.; Polonsky, B.; Steinberg, J.S.; et al. Effect of Carvedilol vs Metoprolol on Atrial and Ventricular Arrhythmias Among Implantable Cardioverter-Defibrillator Recipients. JACC Clin. Electrophysiol. 2023, 9, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Goodfriend, T.L.; Elliott, M.E.; Catt, K.J. Angiotensin Receptors and Their Antagonists. N. Engl. J. Med. 1996, 334, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Makkar, K.M.; Sanoski, C.A.; Spinler, S.A. Role of Angiotensin-Converting Enzyme Inhibitors, Angiotensin II Receptor Blockers, and Aldosterone Antagonists in the Prevention of Atrial and Ventricular Arrhythmias. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 31–48. [Google Scholar] [CrossRef] [PubMed]
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N. Engl. J. Med. 1987, 316, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- SOLVD Investigators; Yusuf, S.; Pitt, B.; Davis, C.E.; Hood, W.B.; Cohn, J.N. Effect of Enalapril on Survival in Patients with Reduced Left Ventricular Ejection Fractions and Congestive Heart Failure. N. Engl. J. Med. 1991, 325, 293–302. [Google Scholar] [CrossRef]
- Pitt, B.; Segal, R.; Martinez, F.A.; Meurers, G.; Cowley, A.J.; Thomas, I.; Deedwania, P.C.; Ney, D.; Snavely, D.B.; Chang, P.I. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 1997, 349, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Poole-Wilson, P.A.; Segal, R.; Martinez, F.A.; Dickstein, K.; Camm, A.J.; Konstam, M.A.; Riegger, G.; Klinger, G.H.; Neaton, J.; et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial—The Losartan Heart Failure Survival Study ELITE II. Lancet 2000, 355, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Östergren, J.; Swedberg, K.; Granger, C.B.; Held, P.; Michelson, E.L.; Olofsson, B.; Yusuf, S.; Pfeffer, M.A. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: The CHARM-Added trial. Lancet 2003, 362, 767–771. [Google Scholar] [CrossRef]
- Cohn, J.N.; Tognoni, G. Valsartan Heart Failure Trial Investigators. A Randomized Trial of the Angiotensin-Receptor Blocker Valsartan in Chronic Heart Failure. N. Engl. J. Med. 2001, 345, 1667–1675. [Google Scholar] [CrossRef]
- AlJaroudi, W.A.; Refaat, M.M.; Habib, R.H.; Al-Shaar, L.; Singh, M.; Gutmann, R.; Bloom, H.L.; Dudley, S.C.; Ellinor, P.T.; Saba, S.F.; et al. Effect of Angiotensin-Converting Enzyme Inhibitors and Receptor Blockers on Appropriate Implantable Cardiac Defibrillator Shock in Patients with Severe Systolic Heart Failure (from the GRADE Multicenter Study). Am. J. Cardiol. 2015, 115, 924–931. [Google Scholar] [CrossRef]
- Schupp, T.; Behnes, M.; Weiß, C.; Nienaber, C.; Lang, S.; Reiser, L.; Bollow, A.; Taton, G.; Reichelt, T.; Ellguth, D.; et al. Prognostic Impact of Angiotensin-Converting Enzyme Inhibitors and Receptor Blockers on Recurrent Ventricular Tachyarrhythmias and Implantable Cardioverter–Defibrillator Therapies. J. Cardiovasc. Pharmacol. 2019, 73, 272–281. [Google Scholar] [CrossRef]
- Schupp, T.; Behnes, M.; Weiß, C.; Nienaber, C.; Lang, S.; Reiser, L.; Bollow, A.; Taton, G.; Reichelt, T.; Ellguth, D.; et al. Beta-Blockers and ACE Inhibitors Are Associated with Improved Survival Secondary to Ventricular Tachyarrhythmia. Cardiovasc. Drugs Ther. 2018, 32, 353–363. [Google Scholar] [CrossRef]
- Garg, R.; Yusuf, S.; Bussmann, W.D.; Sleight, P.; Uprichard, A.; Massie, B.; McGrath, B.; Nilsson, B.; Pitt, B.; Magnani, B.; et al. Overview of Randomized Trials of Angiotensin-Converting Enzyme Inhibitors on Mortality and Morbidity in Patients with Heart Failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995, 273, 1450–1456. [Google Scholar] [CrossRef]
- Flather, M.D.; Yusuf, S.; Køber, L.; Pfeffer, M.; Hall, A.; Murray, G.; Torp-Pedersen, C.; Ball, S.; Pogue, J.; Moyé, L.; et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: A systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet 2000, 355, 1575–1581. [Google Scholar] [CrossRef]
- Tsartsalis, D.; Korela, D.; Karlsson, L.O.; Foukarakis, E.; Svensson, A.; Anastasakis, A.; Venetsanos, D.; Aggeli, C.; Tsioufis, C.; Braunschweig, F.; et al. Risk and Protective Factors for Sudden Cardiac Death: An Umbrella Review of Meta-Analyses. Front. Cardiovasc. Med. 2022, 9, 848021. [Google Scholar] [CrossRef]
- Domanski, M.J.; Exner, D.V.; Borkowf, C.B.; Geller, N.L.; Rosenberg, Y.; Pfeffer, M.A. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction: A meta-analysis of randomized clinical trials. J. Am. Coll. Cardiol. 1999, 33, 598–604. [Google Scholar] [CrossRef]
- Gu, J.; Noe, A.; Chandra, P.; Al-Fayoumi, S.; Ligueros-Saylan, M.; Sarangapani, R.; Maahs, S.; Ksander, G.; Rigel, D.F.; Jeng, A.Y.; et al. Pharmacokinetics and Pharmacodynamics of LCZ696, a Novel Dual-Acting Angiotensin Receptor—Neprilysin Inhibitor (ARNi). J. Clin. Pharmacol. 2010, 50, 401–414. [Google Scholar] [CrossRef]
- Chang, P.-C.; Lin, S.-F.; Chu, Y.; Wo, H.-T.; Lee, H.-L.; Huang, Y.-C.; Wen, M.-S.; Chou, C.-C. LCZ696 Therapy Reduces Ventricular Tachyarrhythmia Inducibility in a Myocardial Infarction-Induced Heart Failure Rat Model. Cardiovasc. Ther. 2019, 2019, 6032631. [Google Scholar] [CrossRef]
- Eiringhaus, J.; Wünsche, C.M.; Tirilomis, P.; Herting, J.; Bork, N.; Nikolaev, V.O.; Hasenfuss, G.; Sossalla, S.; Fischer, T.H. Sacubitrilat reduces pro-arrhythmogenic sarcoplasmic reticulum Ca2+ leak in human ventricular cardiomyocytes of patients with end-stage heart failure. ESC Heart Fail. 2020, 7, 2992–3002. [Google Scholar] [CrossRef]
- Chang, P.-C.; Wo, H.-T.; Lee, H.-L.; Lin, S.-F.; Chu, Y.; Wen, M.-S.; Chou, C.-C. Sacubitril/Valsartan Therapy Ameliorates Ventricular Tachyarrhythmia Inducibility in a Rabbit Myocardial Infarction Model. J. Card. Fail. 2020, 26, 527–537. [Google Scholar] [CrossRef]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef]
- Pfau, D.; Thorn, S.L.; Zhang, J.; Mikush, N.; Renaud, J.M.; Klein, R.; deKemp, R.A.; Wu, X.; Hu, X.; Sinusas, A.J.; et al. Angiotensin Receptor Neprilysin Inhibitor Attenuates Myocardial Remodeling and Improves Infarct Perfusion in Experimental Heart Failure. Sci. Rep. 2019, 9, 5791. [Google Scholar] [CrossRef]
- von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Atar, D.; Krum, H. Angiotensin Receptor Neprilysin Inhibitor LCZ696 Attenuates Cardiac Remodeling and Dysfunction After Myocardial Infarction by Reducing Cardiac Fibrosis and Hypertrophy. Circ. Heart Fail. 2015, 8, 71–78. [Google Scholar] [CrossRef]
- Sung, Y.-L.; Lin, T.-T.; Syu, J.-Y.; Hsu, H.-J.; Lin, K.-Y.; Liu, Y.-B.; Lin, S.-F. Reverse electromechanical modelling of diastolic dysfunction in spontaneous hypertensive rat after sacubitril/valsartan therapy. ESC Heart Fail. 2020, 7, 4040–4050. [Google Scholar] [CrossRef]
- Zile, M.R.; O’Meara, E.; Claggett, B.; Prescott, M.F.; Solomon, S.D.; Swedberg, K.; Packer, M.; McMurray, J.J.; Shi, V.; Lefkowitz, M.; et al. Effects of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients with HFrEF. J. Am. Coll. Cardiol. 2019, 73, 795–806. [Google Scholar] [CrossRef]
- Martens, P.; Nuyens, D.; Rivero-Ayerza, M.; Van Herendael, H.; Vercammen, J.; Ceyssens, W.; Luwel, E.; Dupont, M.; Mullens, W. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin. Res. Cardiol. 2019, 108, 1074–1082. [Google Scholar] [CrossRef]
- de Diego, C.; González-Torres, L.; Núñez, J.M.; Inda, R.C.; Martin-Langerwerf, D.A.; Sangio, A.D.; Chochowski, P.; Casasnovas, P.; Blazquéz, J.C.; Almendral, J. Effects of angiotensin-neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm. 2018, 15, 395–402. [Google Scholar] [CrossRef]
- Rohde, L.E.; Chatterjee, N.A.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Zile, M.; Rouleau, J.; Swedberg, K.; Lefkowitz, M.; et al. Sacubitril/Valsartan and Sudden Cardiac Death According to Implantable Cardioverter-Defibrillator Use and Heart Failure Cause. JACC Heart Fail. 2020, 8, 844–855. [Google Scholar] [CrossRef]
- Curtain, J.P.; Jackson, A.M.; Shen, L.; Jhund, P.S.; Docherty, K.F.; Petrie, M.C.; Castagno, D.; Desai, A.S.; Rohde, L.E.; Lefkowitz, M.P.; et al. Effect of sacubitril/valsartan on investigator-reported ventricular arrhythmias in PARADIGM-HF. Eur. J. Heart Fail. 2022, 24, 551–561. [Google Scholar] [CrossRef]
- Liu, X.-H.; Wang, G.-L.; Xu, Q.; Zhang, L.; Liu, H.-J. Effect of sacubitril/valsartan on the occurrence of cardiac arrhythmias and the risk of sudden cardiac death in heart failure: A meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 943377. [Google Scholar] [CrossRef]
- Pozzi, A.; Abete, R.; Tavano, E.; Kristensen, S.L.; Rea, F.; Iorio, A.; Iacovoni, A.; Corrado, G.; Wong, C. Sacubitril/valsartan and arrhythmic burden in patients with heart failure and reduced ejection fraction: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 28, 1395–1403. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Fernandes, G.C.; Ternes, C.M.; Cardoso, R.; Chaparro, S.V.; Goldberger, J.J. Sacubitril/valsartan versus angiotensin inhibitors and arrhythmia endpoints in heart failure with reduced ejection fraction. Heart Rhythm. O2 2021, 2, 724–732. [Google Scholar] [CrossRef]
- Russo, V.; Bottino, R.; Rago, A.; Papa, A.A.; Liccardo, B.; Proietti, R.; Manna, V.; Golino, P.; D’Onofrio, A.; Nigro, G. The Effect of Sacubitril/Valsartan on Device Detected Arrhythmias and Electrical Parameters among Dilated Cardiomyopathy Patients with Reduced Ejection Fraction and Implantable Cardioverter Defibrillator. J. Clin. Med. 2020, 9, 1111. [Google Scholar] [CrossRef]
- Mujadzic, H.; Prousi, G.S.; Napier, R.; Siddique, S.; Zaman, N. The Impact of Angiotensin Receptor–Neprilysin Inhibitors on Arrhythmias in Patients with Heart Failure: A Systematic Review and Meta-analysis. J. Innov. Card. Rhythm. Manag. 2022, 13, 5164–5175. [Google Scholar] [CrossRef]
- Weber, K.T. Aldosterone in Congestive Heart Failure. N. Engl. J. Med. 2001, 345, 1689–1697. [Google Scholar] [CrossRef]
- Perrier, E.; Kerfant, B.-G.; Lalevee, N.; Bideaux, P.; Rossier, M.F.; Richard, S.; Gómez, A.M.; Benitah, J.-P. Mineralocorticoid Receptor Antagonism Prevents the Electrical Remodeling That Precedes Cellular Hypertrophy After Myocardial Infarction. Circ. 2004, 110, 776–783. [Google Scholar] [CrossRef]
- Ouvrard-Pascaud, A.; Sainte-Marie, Y.; Perrier, R.; Soukaseum, C.; Cat, A.N.D.; Royer, A.; Le Quang, K.; Charpentier, F.; Demolombe, S.; Mechta-Grigoriou, F.; et al. Conditional Mineralocorticoid Receptor Expression in the Heart Leads to Life-Threatening Arrhythmias. Circulation 2005, 111, 3025–3033. [Google Scholar] [CrossRef]
- Brilla, C.G. Aldosterone and myocardial fibrosis in heart failure. Herz 2000, 25, 299–306. [Google Scholar] [CrossRef]
- Cittadini, A.; Monti, M.G.; Isgaard, J.; Casaburi, C.; Strömer, H.; Di Gianni, A.; Serpico, R.; Saldamarco, L.; Vanasia, M.; Saccà, L. Aldosterone receptor blockade improves left ventricular remodeling and increases ventricular fibrillation threshold in experimental heart failure. Cardiovasc. Res. 2003, 58, 555–564. [Google Scholar] [CrossRef]
- Brown, N.J. Eplerenone: Cardiovascular protection. Circulation 2003, 107, 2512–2518. [Google Scholar] [CrossRef]
- Bauersachs, J.; Heck, M.; Fraccarollo, D.; Hildemann, S.K.; Ertl, G.; Wehling, M.; Christ, M. Addition of spironolactone to angiotensin-converting enzyme inhibition in heart failure improves endothelial vasomotor dysfunction: Role of vascular superoxide anion formation and endothelial nitric oxide synthase expression. J. Am. Coll. Cardiol. 2002, 39, 351–358. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Pitt, B.; Williams, G.; Remme, W.; Martinez, F.; Lopez-Sendon, J.; Zannad, F.; Neaton, J.; Roniker, B.; Hurley, S.; Burns, D.; et al. The EPHESUS Trial: Eplerenone in Patients with Heart Failure Due to Systolic Dysfunction Complicating Acute Myocardial Infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc. Drugs Ther. 2001, 15, 79–87. [Google Scholar] [CrossRef]
- Bapoje, S.R.; Bahia, A.; Hokanson, J.E.; Peterson, P.N.; Heidenreich, P.A.; Lindenfeld, J.; Allen, L.A.; Masoudi, F.A. Effects of Mineralocorticoid Receptor Antagonists on the Risk of Sudden Cardiac Death in Patients with Left Ventricular Systolic Dysfunction: A meta-analysis of randomized controlled trials. Circ. Heart Fail. 2013, 6, 166–173. [Google Scholar] [CrossRef]
- Le, H.-H.; El-Khatib, C.; Mombled, M.; Guitarian, F.; Al-Gobari, M.; Fall, M.; Janiaud, P.; Marchant, I.; Cucherat, M.; Bejan-Angoulvant, T.; et al. Impact of Aldosterone Antagonists on Sudden Cardiac Death Prevention in Heart Failure and Post-Myocardial Infarction Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0145958. [Google Scholar] [CrossRef]
- Rossello, X.; Ariti, C.; Pocock, S.J.; Ferreira, J.P.; Girerd, N.; McMurray, J.J.V.; Van Veldhuisen, D.J.; Pitt, B.; Zannad, F. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left-ventricular systolic dysfunction: An individual patient-level meta-analysis of three randomized-controlled trials. Clin. Res. Cardiol. 2018, 108, 477–486. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Ćurčić, I.B.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors—Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M.; Chowdhury, F.I.; Nayan, S.I.; Rahman, M.; Khan, F.; Subhan, N.; et al. Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci. Rep. 2020, 10, 14459. [Google Scholar] [CrossRef]
- Koyani, C.N.; Plastira, I.; Sourij, H.; Hallström, S.; Schmidt, A.; Rainer, P.P.; Bugger, H.; Frank, S.; Malle, E.; von Lewinski, D. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol. Res. 2020, 158, 104870. [Google Scholar] [CrossRef]
- Fu, J.; Xu, H.; Wu, F.; Tu, Q.; Dong, X.; Xie, H.; Cao, Z. Empagliflozin inhibits macrophage inflammation through AMPK signaling pathway and plays an anti-atherosclerosis role. Int. J. Cardiol. 2022, 367, 56–62. [Google Scholar] [CrossRef]
- Byrne, N.J.; Matsumura, N.; Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Darwesh, A.M.; Levasseur, J.L.; Jahng, J.W.S.; Vos, D.; Parajuli, N.; et al. Empagliflozin Blunts Worsening Cardiac Dysfunction Associated with Reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) Inflammasome Activation in Heart Failure. Circ. Heart Fail. 2020, 13, e006277. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Antonio, R.S.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Reboldi, G.; Mengoni, A.; Lauciello, R.; Zuchi, C.; D’Addario, S.; Bardelli, G.; Ambrosio, G. Left ventricular remodeling response to SGLT2 inhibitors in heart failure: An updated meta-analysis of randomized controlled studies. Cardiovasc. Diabetol. 2023, 22, 235. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Zamorano, J.L.; Domingo, M.; Morillas, H.; Nuñez, J.; Marcos, M.C.; Riquelme-Pérez, A.; Teis, A.; Santas, E.; Caro-Martinez, C.; et al. Impact of dapagliflozin on cardiac remodelling in patients with chronic heart failure: The DAPA-MODA study. Eur. J. Heart Fail. 2023, 25, 1352–1360. [Google Scholar] [CrossRef]
- Wu, J.; Liu, T.; Shi, S.; Fan, Z.; Hiram, R.; Xiong, F.; Cui, B.; Su, X.; Chang, R.; Zhang, W.; et al. Dapagliflozin reduces the vulnerability of rats with pulmonary arterial hypertension-induced right heart failure to ventricular arrhythmia by restoring calcium handling. Cardiovasc. Diabetol. 2022, 21, 197. [Google Scholar] [CrossRef]
- Trum, M.; Riechel, J.; Wagner, S. Cardioprotection by SGLT2 Inhibitors—Does It All Come Down to Na+? Int. J. Mol. Sci. 2021, 22, 7976. [Google Scholar] [CrossRef]
- Despa, S.; Bers, D.M. Na+ transport in the normal and failing heart—Remember the balance. J. Mol. Cell Cardiol. 2013, 61, 2–10. [Google Scholar] [CrossRef]
- Wichaiyo, S.; Saengklub, N. Alterations of sodium-hydrogen exchanger 1 function in response to SGLT2 inhibitors: What is the evidence? Heart Fail. Rev. 2022, 27, 1973–1990. [Google Scholar] [CrossRef]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef]
- Mustroph, J.; Wagemann, O.; Lücht, C.M.; Trum, M.; Hammer, K.P.; Sag, C.M.; Lebek, S.; Tarnowski, D.; Reinders, J.; Perbellini, F.; et al. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018, 5, 642–648. [Google Scholar] [CrossRef]
- Nakase, M.; Yahagi, K.; Horiuchi, Y.; Asami, M.; Yuzawa, H.; Komiyama, K.; Tanaka, J.; Aoki, J.; Tanabe, K. Effect of dapagliflozin on ventricular repolarization in patients with heart failure with reduced ejection fraction. Heart Vessel. 2023, 38, 1414–1421. [Google Scholar] [CrossRef]
- Xue, G.; Yang, X.; Zhan, G.; Wang, X.; Gao, J.; Zhao, Y.; Wang, X.; Li, J.; Pan, Z.; Xia, Y. Sodium–Glucose cotransporter 2 inhibitor empagliflozin decreases ventricular arrhythmia susceptibility by alleviating electrophysiological remodeling post-myocardial-infarction in mice. Front. Pharmacol. 2022, 13, 988408. [Google Scholar] [CrossRef]
- Azam, M.A.; Chakraborty, P.; Si, D.; Du, B.; Massé, S.; Lai, P.F.H.; Ha, A.C.T.; Nanthakumar, K. Anti-arrhythmic and inotropic effects of empagliflozin following myocardial ischemia. Life Sci. 2021, 276, 119440. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Curtain, J.P.; Docherty, K.F.; Jhund, P.S.; Petrie, M.C.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur. Heart J. 2021, 42, 3727–3738. [Google Scholar] [CrossRef]
- Fernandes, G.C.; Fernandes, A.; Cardoso, R.; Penalver, J.; Knijnik, L.; Mitrani, R.D.; Myerburg, R.J.; Goldberger, J.J. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021, 18, 1098–1105. [Google Scholar] [CrossRef]
- Sfairopoulos, D.; Zhang, N.; Wang, Y.; Chen, Z.; Letsas, K.P.; Tse, G.; Li, G.; Lip, G.Y.H.; Liu, T.; Korantzopoulos, P. Association between sodium–glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: A meta-analysis of randomized controlled trials. Europace 2022, 24, 20–30. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, H.; Guo, Z. Effect of Sodium-Glucose Co-transporter Protein 2 Inhibitors on Arrhythmia in Heart Failure Patients with or Without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2022, 9, 902923. [Google Scholar] [CrossRef]
- Oates, C.P.; Santos-Gallego, C.G.; Smith, A.; Basyal, B.; Moss, N.; Kawamura, I.; Musikantow, D.R.; Turagam, M.K.; Miller, M.A.; Whang, W.; et al. SGLT2 inhibitors reduce sudden cardiac death risk in heart failure: Meta-analysis of randomized clinical trials. J. Cardiovasc. Electrophysiol. 2023, 34, 1277–1285. [Google Scholar] [CrossRef]
- Fujiki, S.; Iijima, K.; Okabe, M.; Niwano, S.; Tsujita, K.; Naito, S.; Ando, K.; Kusano, K.; Kato, R.; Nitta, J.; et al. Placebo-Controlled, Double-Blind Study of Empagliflozin (EMPA) and Implantable Cardioverter-Defibrillator (EMPA-ICD) in Patients with Type 2 Diabetes (T2DM): Rationale and Design. Diabetes Ther. 2020, 11, 2739–2755. [Google Scholar] [CrossRef]
- Li, H.-L.; Lip, G.Y.H.; Feng, Q.; Fei, Y.; Tse, Y.-K.; Wu, M.-Z.; Ren, Q.-W.; Tse, H.-F.; Cheung, B.-M.Y.; Yiu, K.-H. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2021, 20, 100. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E876–E894. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an Implantable Cardioverter–Defibrillator for Congestive Heart Failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.A.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; et al. Prophylactic Defibrillator Implantation in Patients with Nonischemic Dilated Cardiomyopathy. N. Engl. J. Med. 2004, 350, 2151–2158. [Google Scholar] [CrossRef]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Felker, G.M.; Butler, J.; Ibrahim, N.E.; Piña, I.L.; Maisel, A.; Bapat, D.; Camacho, A.; Ward, J.H.; Williamson, K.M.; Solomon, S.D.; et al. Implantable Cardioverter-Defibrillator Eligibility After Initiation of Sacubitril/Valsartan in Chronic Heart Failure: Insights From PROVE-HF. Circulation 2021, 144, 180–182. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Greene, S.J.; Butler, J.; Filippatos, G.; Lam, C.S.P.; Maggioni, A.P.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Kraigher-Krainer, E.; et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients with Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 2015, 314, 2251–2262. [Google Scholar] [CrossRef]
- Hulot, J.-S.; Trochu, J.-N.; Donal, E.; Galinier, M.; Logeart, D.; De Groote, P.; Juillière, Y. Vericiguat for the treatment of heart failure: Mechanism of action and pharmacological properties compared with other emerging therapeutic options. Expert Opin. Pharmacother. 2021, 22, 1847–1855. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Chen, T.; Kong, B.; Shuai, W.; Gong, Y.; Zhang, J.; Huang, H. Vericiguat alleviates ventricular remodeling and arrhythmias in mouse models of myocardial infarction via CaMKII signaling. Life Sci. 2023, 334, 122184. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Diaz, R.; Felker, G.M.; McMurray, J.J.V.; Metra, M.; Solomon, S.D.; Adams, K.F.; Anand, I.; Arias-Mendoza, A.; Biering-Sørensen, T.; et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Drucker, D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of Liraglutide on Clinical Stability Among Patients with Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef]
- Neves, J.S.; Vasques-Nóvoa, F.; Borges-Canha, M.; Leite, A.R.; Sharma, A.; Carvalho, D.; Packer, M.; Zannad, F.; Leite-Moreira, A.; Ferreira, J.P. Risk of adverse events with liraglutide in heart failure with reduced ejection fraction: A post hoc analysis of the FIGHT trial. Diabetes Obes. Metab. 2023, 25, 189–197. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—A multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef]
- Wu, S.; Lu, W.; Chen, Z.; Dai, Y.; Chen, K.; Zhang, S. Association of glucagon-like peptide-1 receptor agonists with cardiac arrhythmias in patients with type 2 diabetes or obesity: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2022, 14, 195. [Google Scholar] [CrossRef]
- Al-Sadawi, M.A.; Aslam, F.M.; Tao, M.; Alsaiqali, M.; Almasry, I.O.; Fan, R.; Rashba, E.J.; Singh, A. Effects of GLP-1 Agonists on mortality and arrhythmias in patients with Type II diabetes. IJC Heart Vasc. 2023, 47, 101218. [Google Scholar] [CrossRef]
- Bizino, M.B.; Jazet, I.M.; Westenberg, J.J.M.; van Eyk, H.J.; Paiman, E.H.M.; Smit, J.W.A.; Lamb, H.J. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: Randomized placebo-controlled trial. Cardiovasc. Diabetol. 2019, 18, 55. [Google Scholar] [CrossRef]
- Durak, A.; Turan, B. Liraglutide provides cardioprotection through the recovery of mitochondrial dysfunction and oxidative stress in aging hearts. J. Physiol. Biochem. 2023, 79, 297–311. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
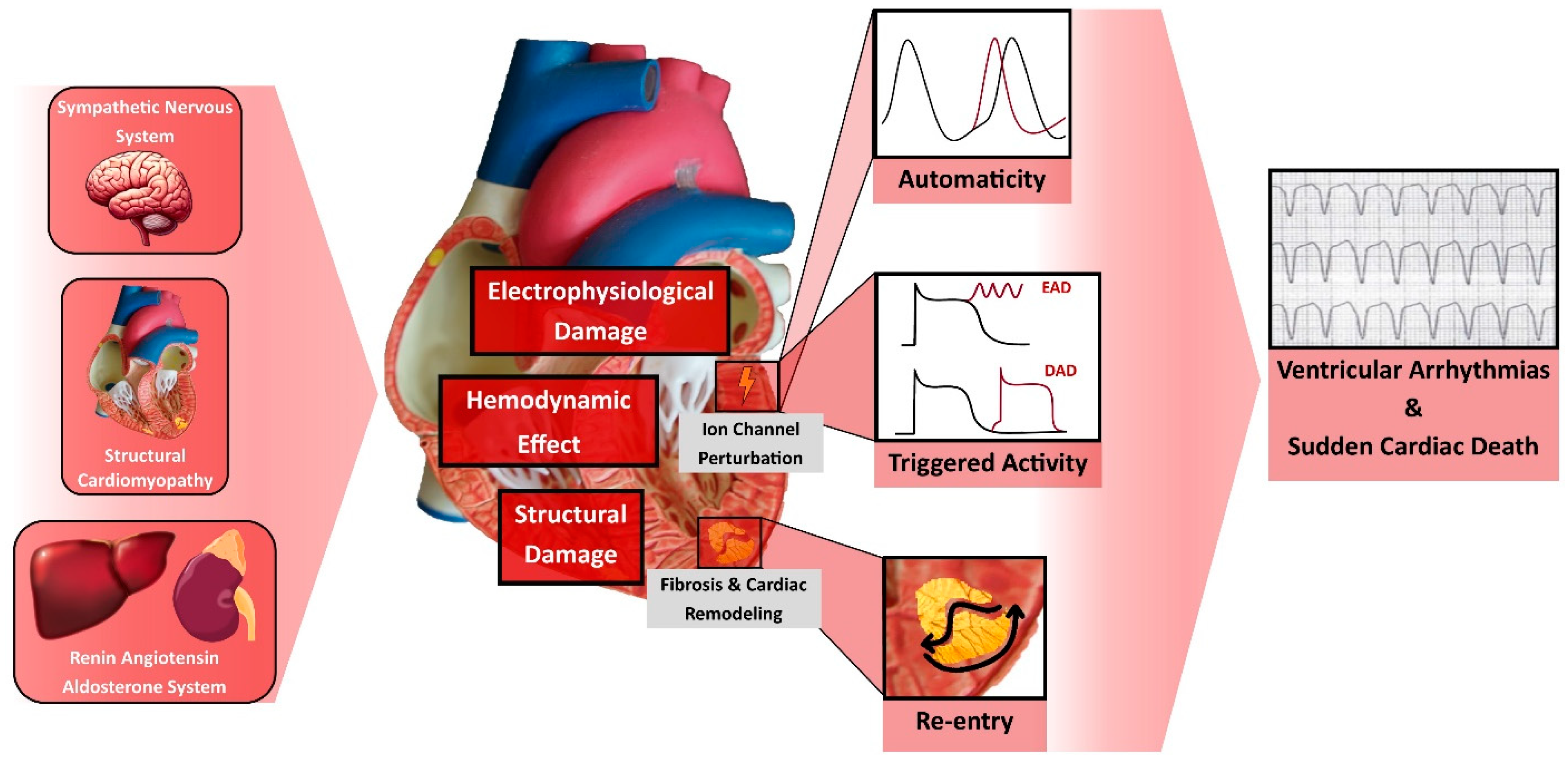
Figure 1.
Pathophysiology of Ventricular Arrhythmia and Sudden Cardiac Death in Heart Failure. DAD, Delayed Afterdepolarization; EAD, Early Afterdepolarization.
Figure 1.
Pathophysiology of Ventricular Arrhythmia and Sudden Cardiac Death in Heart Failure. DAD, Delayed Afterdepolarization; EAD, Early Afterdepolarization.
Figure 2.
Effects of Guideline-directed medical therapy on Ventricular Arrhythmia and Sudden Cardiac Death. ACEi, Angiotensin-Converting Enzyme Inhibitors; ARB, Angiotensin II Receptor Blocker; ARNi, Angiotensin Receptor–Neprilysin Inhibitors; DAD, Delayed Afterdepolarization; EAD, Early Afterdepolarization; GLP-1 RA, Glucagon-like peptide 1 receptor agonists; MRA, Mineralocorticoid Receptor Antagonist; SGLT2i, Sodium-Glucose Cotransporter 2 Inhibitor.
Figure 2.
Effects of Guideline-directed medical therapy on Ventricular Arrhythmia and Sudden Cardiac Death. ACEi, Angiotensin-Converting Enzyme Inhibitors; ARB, Angiotensin II Receptor Blocker; ARNi, Angiotensin Receptor–Neprilysin Inhibitors; DAD, Delayed Afterdepolarization; EAD, Early Afterdepolarization; GLP-1 RA, Glucagon-like peptide 1 receptor agonists; MRA, Mineralocorticoid Receptor Antagonist; SGLT2i, Sodium-Glucose Cotransporter 2 Inhibitor.
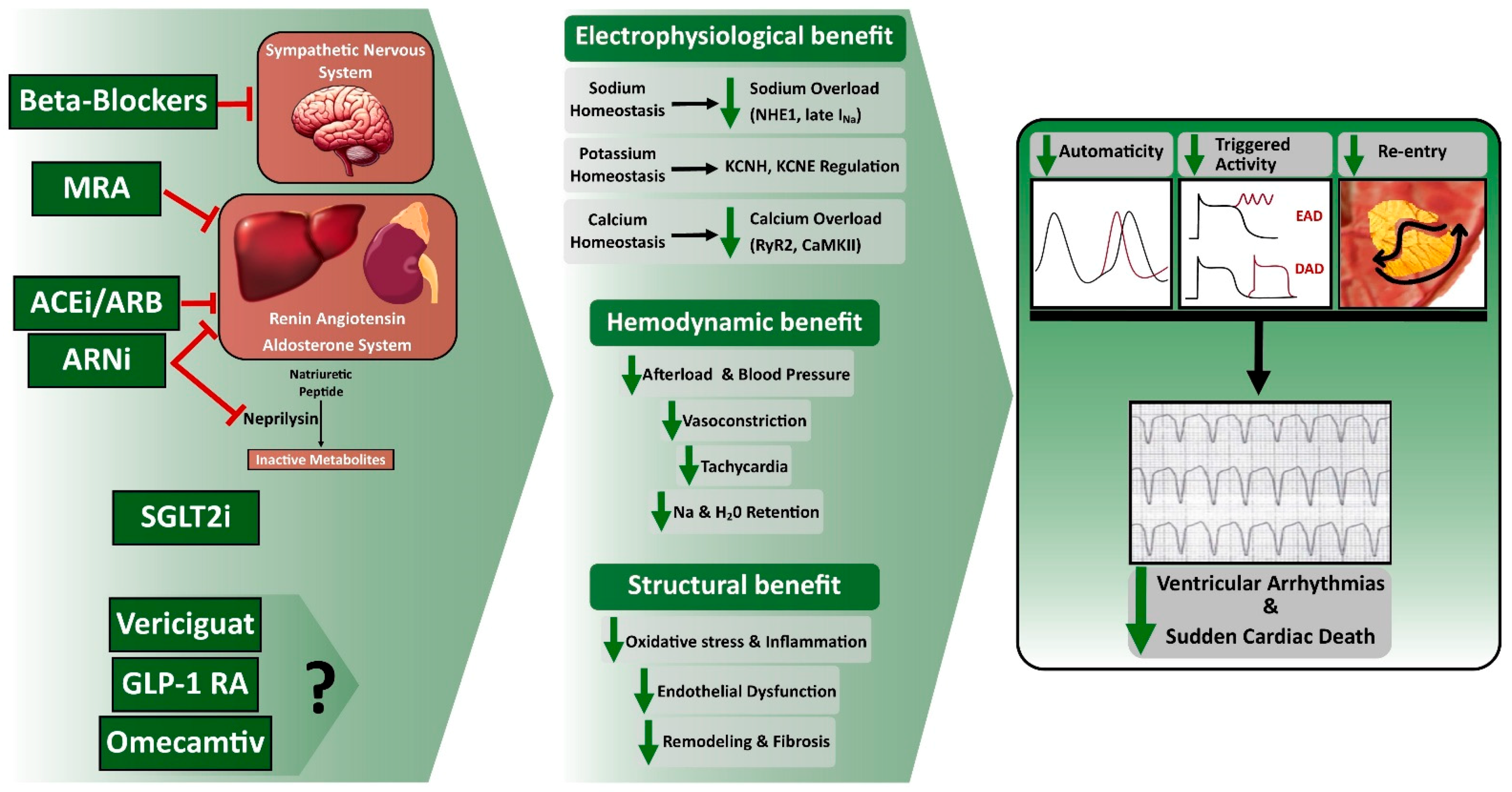
Table 1.
Major studies and meta-analyses regarding effect of ARNi on ventricular arrythmia and sudden cardiac death.
Table 1.
Major studies and meta-analyses regarding effect of ARNi on ventricular arrythmia and sudden cardiac death.
| Authors Journal Year |
Type of Study Intervention |
No. of Patients in the Population |
Effect on VA and SCD |
|---|---|---|---|
| Martens, et al. Clin Res Cardiol. 2019 [57]. |
Retrospective, cohort Pre- vs. Post-ARNi initiation |
151 HFrEF with ICD |
VA reduction (51 vs. 14; p < 0.001) ICD shock reduction (16 vs. 6; p < 0.001) |
| De Diego, et al. Heart Rhythm. 2018 [58]. |
Prospective, cohort ARNi vs. ACEi/ARB |
240 HFrEF with ICD |
VA and ICD shock reduction (0.8% vs. 6.7%; p < 0.02) |
| Russo, et al. J Clin Med. 2020 [64]. |
Prospective, cohort ARNi |
167 HFrEF with ICD |
VA reduction (15 vs. 4; p = 0.03) ICD shock reduction (13 vs. 3; p = 0.02) |
| Rohde et al. JACC Heart Fail. 2020 [59]. |
RCT—post hoc analysis ARNi vs. ACEi |
8399 HFrEF |
SCD reduction in the ICD group (HR 0.49; 95% CI 0.25–0.99) and non-ICD group (HR 0.81; 95% CI 0.67–0.98) |
| Curtain, et al. Eur J Heart Fail. 2022 [60]. |
RCT—post hoc analysis ARNi vs. ACEi |
8399 HFrEF |
VA reduction (HR 0.76; 95% CI 0.62–0.95) |
| Fernandes, et al. Heart Rhythm O2. 2021 [63]. |
Meta-analysis ARNi vs. ACEi/ARB |
11,204 HFrEF |
SCD reduction (OR 0.78; 95% CI 0.63–0.96) VA reduction (OR 0.45; 95% CI 0.25–0.79) Higher BiV Pacing (p < 0.0001) |
| Liu, et al. Front Cardiovasc Med. 2022 [61]. |
Meta-analysis ARNi vs. ACEi/ARB/Placebo |
18,500 HFrEF or HFpEF |
No VA reduction (RR 0.86; 95% CI 0.68–1.10) SCD reduction (RR 0.79; 95% CI 0.70–0.90) |
| Mujadzic, et al. J Innoc Card Rhythm Mang. 2022 [65]. |
Meta-analysis ARNi vs. ACEi/ARB/Placebo |
18,548 HFrEF or HFpEF |
VA & SCD reduction (OR 0.71; 95% CI 0.54–0.93) ICD shock reduction (OR 0.23; 95% CI 0.11–0.47) |
| Pozzi, et al. Heart Fail Rev. 2023 [62]. |
Meta-analysis ARNi vs. ACEi/ARB |
8837 HFrEF |
VA reduction (OR 0.78; 95% CI 0.63–0.96 for RCT and RR 0.62; 95% CI 0.53–0.72 for observational studies) ICD shock reduction (RR 0.24; 95% CI 0.12–0.24) |
Table 2.
Major studies and meta-analyses regarding the effect of SGLT2i on ventricular arrhythmia and sudden cardiac death.
Table 2.
Major studies and meta-analyses regarding the effect of SGLT2i on ventricular arrhythmia and sudden cardiac death.
| Authors Journal Year |
Type of Study Intervention |
No. of Patients in the Population |
Effect on VA and SCD |
|---|---|---|---|
| Curtain, et al. Eur Heart J. 2021 [98]. |
RCT—post hoc analysis Dapaglifozin vs. Placebo |
4744 HFrEF |
Reduction in the composite outcome of VA and SCD (HR 0.79; 95% CI 0.63–0.99) |
| Fernandes, et al. Heart Rhythm. 2021 [99]. |
Meta-analysis SGLT2i vs. Placebo |
63,166 T2DM or HF |
SCD reduction (HR 0.72; 95% CI 0.54–0.97) No difference in VA |
| Li, et al. Cardiovasc Diabetol. 2021 [104]. |
Meta-analysis SGLT2i vs. Placebo |
52,115 T2DM or CKD or HF |
VA reduction (RR 0.73; 95% CI 0.53–0.99) |
| Sfairopoulos et al. Europace. 2022 [100]. |
Meta-analysis SGLT2i vs. Placebo |
55,590 T2DM or CKD or HF |
No VA reduction (RR 0.84; 95% CI 0.66–1.06) No SCD reduction (RR 0.74; 95% CI 0.50–1.08) |
| Yin et al. Front Cardiovasc Med. 2022 [101]. |
Meta-analysis SGLT2i vs. Placebo |
10,344 HFrEF or HFpEF |
No VA reduction (VT: RR 0.90; 95% CI 0.44–1.82; VF: RR 1.40; 95% CI 0.73–2.67) |
| Oates, et al. J Cardiovasc Electrophysiol. 2023 [102]. |
Meta-analysis SGLT2i vs. Placebo |
10,796 HFrEF or HFpEF |
SCD reduction (RR 0.68; 95% CI 0.48–0.95) No VA reduction (RR 1.03; 95% CI 0.83–1.29) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
[ad_2]