JPM | Free Full-Text | Posterior Tibial Nerve Stimulation for the Treatment of Detrusor Overactivity in Multiple Sclerosis Patients: A Narrative Review
[ad_1]
1. Introduction
2. Multiple Sclerosis and Bladder Function
3. Methods
A literature search was conducted on three databases (Medline through PubMed, Web of Science and Scopus). Targeted keywords, such as multiple sclerosis, bladder dysfunction, PTNS and neurogenic detrusor overactivity were deployed. Additionally, a meticulous scrutiny of reference lists from related articles was undertaken, reinforcing the commitment to a comprehensive exploration of the existing literature.
Inclusion criteria focused on studies involving adults with bladder disorders related to multiple sclerosis (MS). Studies that provided detailed descriptions of specific rehabilitation treatments were specifically sought. Furthermore, we included only research designs that were carefully chosen, including published prospective and retrospective studies as well as randomized controlled trials (RCTs). It was imperative that studies met these criteria for inclusion in our analysis. Furthermore, the language criterion was restricted to original English text to maintain consistency in data interpretation.
To refine our focus, we implemented specific exclusion criteria. Studies related to neurological diseases other than MS were excluded, as the emphasis of this narrative review was on the unique challenges presented by MS-related bladder disorders. Studies that did not explicitly address urinary symptoms were also excluded from our analysis. Furthermore, to eliminate redundancy and maintain the integrity of our findings, duplicate studies were meticulously excluded from consideration.
4. Posterior Tibial Nerve Simulation
5. PTNS Is an Effective Treatment for Detrusor Overactivity in MS Patients
5.1. PTNS Efficacy for Detrusor Overactivity Treatment in MS Patients
However, data for progressive neurologic conditions like MS remain limited, with only a handful of studies showcasing the efficacy of such treatments in MS patients.
5.2. Long-Term PTNS Effects and Combination Treatments
5.3. PTNS Prognostic Indicators and Alternative Approaches
There is a scarcity of data concerning PTNS efficacy in managing DU and DSD. Given the limited evidence available, we are unable to recommend these methods for MS patients. Further research is warranted to ascertain their potential benefits and safety profile in this population.
6. Financial Aspects of PTNS in Modern Health-Care Systems
7. Anticipating Future Enhancements and Evolutions in PTNS Therapy
7.1. Is There Any Place for Conditional Stimulation?
Inhibition of detrusor contraction is necessary in the event of an involuntary contraction. PTNS could be introduced when intravesical pressure begins to rise and ceased when pressure returns to normal levels. This approach is termed conditional or event-triggered stimulation.
7.2. Implantable Devices for PTNS in MS Patients
7.3. Future Perspectives
The majority of PTNS research has adhered to a standardized protocol: 10 to 12 weekly sessions lasting 30 min each, with preset and relatively fixed stimulation parameters and the insertion of only one needle at a time. However, altering the treatment regimen or stimulation parameters could potentially yield different, potentially improved outcomes. This includes exploring bilateral therapy instead of unilateral. An accelerated stimulation schedule offers the advantage of achieving clinical results more rapidly. In terms of stimulation parameters, there is general consensus that pulse intensity should be set to a well-tolerated level, with studies suggesting that frequencies below 20 Hz may yield better results, particularly frequencies as low as 5–6 Hz. Similarly, adjustments in pulse duration, where the standard setting for PTNS is 0.2 milliseconds, could also impact outcomes.
8. PTNS Side Effects and Limitations
9. Conclusions
In wrapping up, the pervasive issue of bladder dysfunction significantly affects the life quality of nearly 80% of individuals living with MS, highlighting the need for alternative therapeutic strategies beyond conventional methods. This narrative review brings to light the potential of PTNS as an innovative solution for improving bladder control and overall life satisfaction among the MS community. The compilation of various study findings presents PTNS as an effective measure, particularly when the peripheral tibial nerve is stimulated for 20 min daily across a three-month span, yielding consistent improvements and high acceptance among patients. Notably, while some research indicates the absence of immediate benefits from electrical stimulation of the posterior tibial nerve, extended PTNS sessions have been linked to ongoing enhancements in symptoms related to the lower urinary tract. The significant effectiveness and patient receptiveness towards PTNS highlight its viability as either an independent or supplementary therapy for managing neurogenic bladder issues in MS sufferers.
Despite these positive outcomes, the call for additional, varied research remains, especially to understand its effects on specific groups like men with idiopathic OAB. The evidence suggests that PTNS could offer relief to individuals with OAB syndrome unresponsive to conventional treatments, further supported by its commendable safety profile, with negligible adverse effects reported. Nonetheless, the adoption of PTNS should be viewed as an adjunct rather than a cure-all for bladder issues within the MS demographic. The choice to implement PTNS ought to stem from an in-depth consultation with medical practitioners, meticulously balancing its advantages against possible constraints and tailored to the unique circumstances of each patient, including the severity of symptoms and overall health condition. As ongoing research progresses, PTNS is poised to become a key player in the tailored management of bladder dysfunction in MS, promising to elevate patient care standards and personalize treatment pathways.
Author Contributions
Conceptualization, V.S. and A.Z. (Athanasios Zachariou); writing—original draft preparation, V.S., A.Z. (Athanasios Zikopoulos) and S.T.; writing—review and editing, D.Z., I.G. and A.K.; supervision, B.S., N.S. and A.Z. (Athanasios Zachariou); project administration, A.Z. (Athanasios Zachariou). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar]
- Panicker, J.N. Neurogenic bladder: Epidemiology, diagnosis, and management. Semin. Neurol. 2020, 40, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Bhide, A.A.; Tailor, V.; Fernando, R.; Khullar, V.; Digesu, G.A. Posterior tibial nerve stimulation for overactive bladder-techniques and efficacy. Int. Urogynecol. J. 2020, 31, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, M.; Chiaramonte, R.; Di Benedetto, P. Management of bladder dysfunction in multiple sclerosis: A systematic review and meta-analysis of studies regarding bladder rehabilitation. Eur. J. Phys. Rehabil. Med. 2022, 58, 387–396. [Google Scholar] [CrossRef]
- Pericolini, M.; Miget, G.; Hentzen, C.; Finazzi Agrò, E.; Chesnel, C.; Lagnau, P.; Haddad, R.; Grasland, M.; Amarenco, G. Cortical, spinal, sacral, and peripheral neuromodulations as therapeutic approaches for the treatment of lower urinary tract symptoms in multiple sclerosis patients: A review. Neuromodulation 2022, 25, 1065–1075. [Google Scholar] [CrossRef]
- Pang, D.; Gao, Y.; Liao, L. Functional brain imaging and central control of the bladder in health and disease. Front. Physiol. 2022, 13, 914963. [Google Scholar] [CrossRef]
- Aharony, S.M.; Lam, O.; Corcos, J. Evaluation of lower urinary tract symptoms in multiple sclerosis patients: Review of the literature and current guidelines. Can. Urol. Assoc. J. 2017, 11, 61–64. [Google Scholar] [CrossRef]
- Rahnama’i, S.M. Neuromodulation for functional bladder disorders in patients with multiple sclerosis. Mult. Scler. 2020, 26, 1274–1280. [Google Scholar] [CrossRef]
- Tornic, J.; Panicker, J.N. The Management of Lower Urinary Tract Dysfunction in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2018, 18, 54. [Google Scholar] [CrossRef]
- Peyronnet, B.; Krupp, L.B.; Reynolds, W.S.; Game, X.; Amaernco, G.; Cornu, J.N.; Ryerson, L.Z.; Sammarco, C.L.; Howard, J.E.; Charlson, R.W.; et al. Nocturia in patients with multiple sclerosis. Rev. Urol. 2019, 21, 63–73. [Google Scholar] [PubMed]
- Bøe Lunde, H.M.; Aae, T.F.; Indrevåg, W.; Bjorvatn, B.; Myhr, K.M.M.; Bø, L. Poor sleep in patients with multiple sclerosis. PLoS ONE 2012, 7, e49996. [Google Scholar] [CrossRef] [PubMed]
- Pokryszko-Dragan, A.; Bilińska, M.; Gruszka, E.; Biel, Ł.; Kamińska, K.; Konieczna, K. Sleep disturbances in patients with multiple sclerosis. Neurol. Sci. 2013, 34, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Zecca, C.; Riccitelli, G.C.; Disanto, G.; Singh, A.; Digesu, G.A.; Puccini, F.; Mattioli, M.; Tubaro, A.; Gobbi, C. Urinary incontinence in multiple sclerosis: Prevalence, severity and impact on patients’ quality of life. Eur. J. Neurol. 2016, 23, 1228–1234. [Google Scholar] [CrossRef]
- Sapouna, V.; Thanopoulou, S.; Papriakas, D.; Papakosta, S.; Sakopoulou, M.; Zachariou, D.; Zikopoulos, A.; Kaltsaw, A.; Vrachnis, N.; Vrachnis, D.; et al. Pelvic floor muscle training and its benefits for multiple sclerosis patients suffering from urinary incontinence and sexual dysfunction. Cureus 2023, 15, e47086. [Google Scholar] [CrossRef] [PubMed]
- Abello, A.; Badin, J.; Das, A.K. Worsening disability status in multiple sclerosis predicts urologic complications. Int. Urol. Nephrol. 2020, 52, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Bientinesi, R.; Gandi, C.; Bassi, P. Managing Urological Disorders in Multiple Sclerosis Patients: A Review of Available and Emerging Therapies. Int. Neurourol. J. 2020, 24, 118–126. [Google Scholar] [CrossRef] [PubMed]
- McGuire, E.J.; Zhang, S.C.; Horwinski, E.R.; Lytton, B. Treatment of motor and sensory detrusor instability by electrical stimulation. J. Urol. 1983, 129, 78–79. [Google Scholar] [CrossRef]
- Stoller, M.L. Afferent nerve stimulation for pelvic floor dysfunction. Eur. Urol. 1999, 35 (Suppl. S2), 132. [Google Scholar]
- Carilli, M.; Pacini, P.; Serati, M.; Iacovelli, V.; Bianchi, D.; Petta, F.; Pastore, S.; Amato, I.; Fede Spicchiale, C.; D’Ippolito, G.; et al. Percutaneous tibial nerve stimulation in the treatment of neurogenic detrusor overactivity in multiple sclerosis patients: A historically controlled study. Ther. Adv. Urol. 2023, 15, 17562872231177779. [Google Scholar] [CrossRef] [PubMed]
- Finazzi-Agro, E.; Rocchi, C.; Pachatz, C.; Petta, F.; Sperra, E.; Mori, F.; Sciobica, F.; Marfia, G.A. Percutaneous tibial nerve stimulation produces effects on brain activity: Study on the modifications of the long latency somatosensory evoked potentials. Neurourol. Urodyn. 2009, 28, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.P. The electrical control of sphincter incompetence. Lancet 1963, 2, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Fall, M.; Lindstrom, S. Electrical stimulation: A physiologic approach to the treatment of urinary incontinence. Urol. Clin. N. Am. 1991, 18, 393–407. [Google Scholar] [CrossRef]
- Danişman, A.; Kutlu, Ö.; Akkaya, E.; Karpuzoğlu, G.; Erdoğru, T. Tibial nerve stimulation diminishes mast cell infiltration in the bladder wall induced by interstitial cystitis urine. Scand. J. Urol. Nephrol. 2007, 41, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Huang, S.T.; Hsu, K.; Lin, A.; Stoller, M.L.; Lue, T.F. Electroacupuncture decreases c-fos expression in the spinal cord induced by noxious stimulation of the rat bladder. J. Urol. 1998, 160, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- McPhail, C.; Carey, R.; Nambiar, S.; Willison, N.; Bahadori, S.; Aryan, P.; Nguyen, T.; Behnia-Willison, F. The Investigation of Percutaneous Tibial Nerve Stimulation (PTNS) as a Minimally Invasive, Non-Surgical, Non-Hormonal Treatment for Overactive Bladder Symptoms. J. Clin. Med. 2023, 12, 3490. [Google Scholar] [CrossRef] [PubMed]
- Gaziev, G.; Topazio, L.; Iacovelli, V.; Asimakopoulos, A.; Di Santo, A.; De Nunzio, C.; Finazzi-Agrò, E. Percutaneous Tibial Nerve Stimulation (PTNS) efficacy in the treatment of lower urinary tract dysfunctions: A systematic review. BMC Urol. 2013, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jian, Z.; Ma, Y.; Jin, X.; Li, H.; Wang, K. Percutaneous tibial nerve stimulation for overactive bladder syndrome: A systematic review and meta-analysis. Int. Urogynecol. J. 2020, 31, 2457–2471. [Google Scholar] [CrossRef]
- Marzouk, M.H.; Darwish, M.H.; El-Tamawy, M.S.; Morsy, S.; Abbas, R.L.; Ali, A.S. Posterior tibial nerve stimulation as a neuromodulation therapy in treatment of neurogenic overactive bladder in multiple sclerosis: A prospective randomized controlled study. Mult. Scler. Relat. Disord. 2022, 68, 104252. [Google Scholar] [CrossRef]
- Kabay, S.; Kabay, S.C.; Yucel, M.; Ozden, H.; Yilmaz, Z.; Aras, O.; Bahar, A. The clinical and urodynamic results of a three months percutaneous posterior tibial nerve stimulation treatment in patients with multiple sclerosis-related neurogenic bladder dysfunction. Neurourol. Urodyn. 2009, 28, 965–968. [Google Scholar]
- Zecca, C.; Digesu, G.A.; Robshaw, P.; Singh, A.; Elneil, S.; Gobbi, C. Maintenance percutaneous posterior nerve stimulation for refractory lower urinary tract symptoms in patients with multiple sclerosis: An open label, multicenter, prospective study. J. Urol. 2014, 191, 697–702. [Google Scholar] [CrossRef]
- Kabay, S.C.; Kabay, S.; Mestan, E.; Cetiner, M.; Ayas, S.; Sevim, M.; Ozden, H.; Ozisik Karaman, H. Long term sustained therapeutic effects of percutaneous posterior tibial nerve stimulation treatment of neurogenic overactive bladder in multiple sclerosis patients: 12-months results. Neurourol. Urodyn. 2017, 36, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Tudor, K.I.; Seth, J.H.; Liechti, M.D.; Ochulor, J.; Gonzales, G.; Haslam, C.; Fox, Z.; Pakzad, M.; Panicker, J.N. Outcomes following percutaneous tibial nerve stimulation (PTNS) treatment for neurogenic and idiopathic overactive bladder. Clin. Auton. Res. 2020, 30, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Kobberø, H.; Pedersen, T.B.; Poulsen, M.H. Percutaneous tibial nerve stimulation for idiopathic and neurogenic overactive bladder dysfunction: A four-year follow-up single-centre experience. Scand. J. Urol. 2021, 55, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, N.; Orakifar, N.; Kouti, L.; Shamsaei, G.; Seyedtabib, M.; Jafari, M. Solifenacin versus posterior tibial nerve stimulation for overactive bladder in patients with multiple sclerosis. Front. Neurosci. 2023, 17, 1107886. [Google Scholar] [CrossRef] [PubMed]
- Al-Danakh, A.; Safi, M.; Alradhi, M.; Almoiligy, M.; Chen, Q.; Al-Nusaif, M.; Yang, X.; Al-Dherasi, A.; Zhu, X.; Yang, D. Posterior tibial nerve stimulation for overactive bladder: Mechanism, classification, and management outlines. Park. Dis. 2022, 2022, 2700227. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.E.; Menefee, S.A.; Diwadkar, G.B. 8 versus 12 weeks of percutaneous tibial nerve stimulation and response predictors for overactive bladder. Int. Urogynecol. J. 2020, 31, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Kabay, S.C.; Yucel, M.; Kabay, S. Acute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: Urodynamic study. Urology 2008, 71, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, A.; D’ancona, C.A.; Perissinotto, M.C.; McLean, L.; Damasceno, B.P.; de Moraes Lopes, M.H. Pelvic Floor Muscle Training with and without Electrical Stimulation in the Treatment of Lower Urinary Tract Symptoms in Women with Multiple Sclerosis. J. Wound Ostomy Cont. Nurs. 2016, 43, 414–419. [Google Scholar] [CrossRef]
- de Sèze, M.; Raibaut, P.; Gallien, P.; Even-Schneider, A.; Denys, P.; Bonniaud, V.; Gamé, X.; Amarenco, G. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: Results of a multicenter prospective study. Neurourol. Urodyn. 2011, 30, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Jacomo, R.H.; Alves, A.Τ.; Lucio, A.; Garcia, P.A.; Lorena, D.C.R.; de Sousa, J.B. Transcutaneous tibial nerve stimulation versus parasacral stimulation in the treatment of overactive bladder in elderly people: A triple-blinded randomized controlled trial. Clinics 2020, 75, e1477. [Google Scholar] [CrossRef] [PubMed]
- Ramırez-Garcıa, Ι.; Blanco-Ratto, L.; Kauffmann, S.; Carralero-Martınez, A.; Emılia, S. Efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome: Randomized control trial. Neurourol. Urodyn. 2019, 38, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.A.; Clinch, J.; Radhakrishnan, M.; Healy, A.; McMillan, V.; Morris, E.; Rua, Τ.; Ofuya, Μ.; Wang, Y.; Dimmock, P.W.; et al. The geko electro-stimulation device for venous thromboembolism prophylaxis: A NICE medical technology guidance. Appl. Health Econ. Health Policy 2015, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Kurdoğlu, Z.; Carr, D.; Harmouche, J.; Ünlü, S.; Kılıç, G.S. Short-term results of the efficacy of percutaneous tibial nerve stimulation on urinary symptoms and its financial cost. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 7–10. [Google Scholar] [CrossRef]
- Staskin, D.R.; Peters, K.M.; MacDiarmid, S.; Shore, N.; de Groat, W.C. Percutaneous tibial nerve stimulation: A clinically and cost-effective addition to the overactive bladder algorithm of care. Curr. Urol. Rep. 2012, 13, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Bercik, R.S.; Werner, E.F.; Thung, S.F. Cost-effectiveness of percutaneous tibial nerve stimulation versus extended release tolterodine for overactive bladder. J. Urol. 2012, 187, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Martinson, M.; MacDiarmid, S.; Black, E. Cost of neuromodulation therapies for overactive bladder: Percutaneous tibial nerve stimulation versus sacral nerve stimulation. J. Urol. 2013, 189, 210–216. [Google Scholar] [CrossRef]
- Sirls, E.R.; Killinger, K.A.; Boura, J.A.; Peters, K.M. Percutaneous tibial nerve stimulation in the office setting: Real-world experience of over 100 patients. Urology 2018, 113, 34–39. [Google Scholar] [CrossRef]
- Fjorback, M.; van Rey, F.; van der Pal, F.; Rijkhoff, N.; Petersen, T.; Heesakkers, J. Acute Urodynamic Effects of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with MS. Eur. Urol. 2007, 51, 464–472. [Google Scholar] [CrossRef]
- Amarenco, G.; Ismael, S.S.; Even-Schneider, A.; Demaille-Wlodyka, S.; Parratte, B.; Kerdraon, J. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J. Urol. 2003, 169, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Heesakkers, J.P.F.A.; Digesu, G.A.; van Breda, J.; Van Kerrebroeck, P.; Elneil, S. A novel leadless, miniature implantable Tibial Nerve Neuromodulation System for the management of overactive bladder complaints. Neurourol. Urodyn. 2018, 37, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- van Breda, H.M.K.; Martens, F.M.J.; Tromp, J.; Heesakkers, J.P.F.A. A New Implanted Posterior Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome: 3-Month Results of a Novel Therapy at a Single Center. J. Urol. 2017, 198, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Dorsthorst, M.J.T.; Digesu, G.A.; Tailor, V.; Gore, M.; van Kerrebroeck, P.E.; van Breda, H.M.K.; Elneil, S.; Heesakkers, J.P.F.A. 3-Year Followup of a New Implantable Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome. J. Urol. 2020, 204, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Negron, J.M.; Goldman, H.B. New devices and technologies for the management of overactive bladder. Curr. Urol. Rep. 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Maino, P.; Koetsier, E.; Disanto, G.; Renard, J.; Digesu, A.; Gobbi, C.; Zecca, C. Efficacy and safety of the implantable, magnetic resonance imaging-compatible StimRouter neuromodulation system in the treatment of refractory lower urinary tract symptoms in multiple sclerosis patients. Eur. J. Neurol. 2024, 31, e16146. [Google Scholar] [CrossRef]
- Gilling, P.; Meffan, P.; Kaaki, B.; MacDiarmid, S.; Lucente, V.; Clark, M.; Sen, A.K.; English, S.; Sand, P.K. Twelve-month Durability of a Fully-implanted, Nickel-sized and Shaped Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome with Urgency Urinary Incontinence: A Single-Arm, Prospective study. Urology 2021, 157, 71–78. [Google Scholar] [CrossRef]
- MacDiarmid, S.; Staskin, D.R.; Lucente, V.; Kaaki, B.; English, S.; Gilling, P.; Meffan, P.; Clark, M.; Sand, P.K.; Sen, S.K.; et al. Feasibility of a Fully Implanted, Nickel Sized and Shaped Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome with Urgency Urinary Incontinence. J. Urol. 2019, 201, 967–972. [Google Scholar] [CrossRef]
- Rogers, A.; Bragg, S.; Ferrante, K.; Chuladatta, T.; Peterson, D.K.L. Pivotal Study of Leadless Tibial Nerve Stimulation with eCoin® for Urgency Urinary Incontinence: An Open-Label, Single Arm Trial. J. Urol. 2021, 206, 399–408. [Google Scholar] [CrossRef]
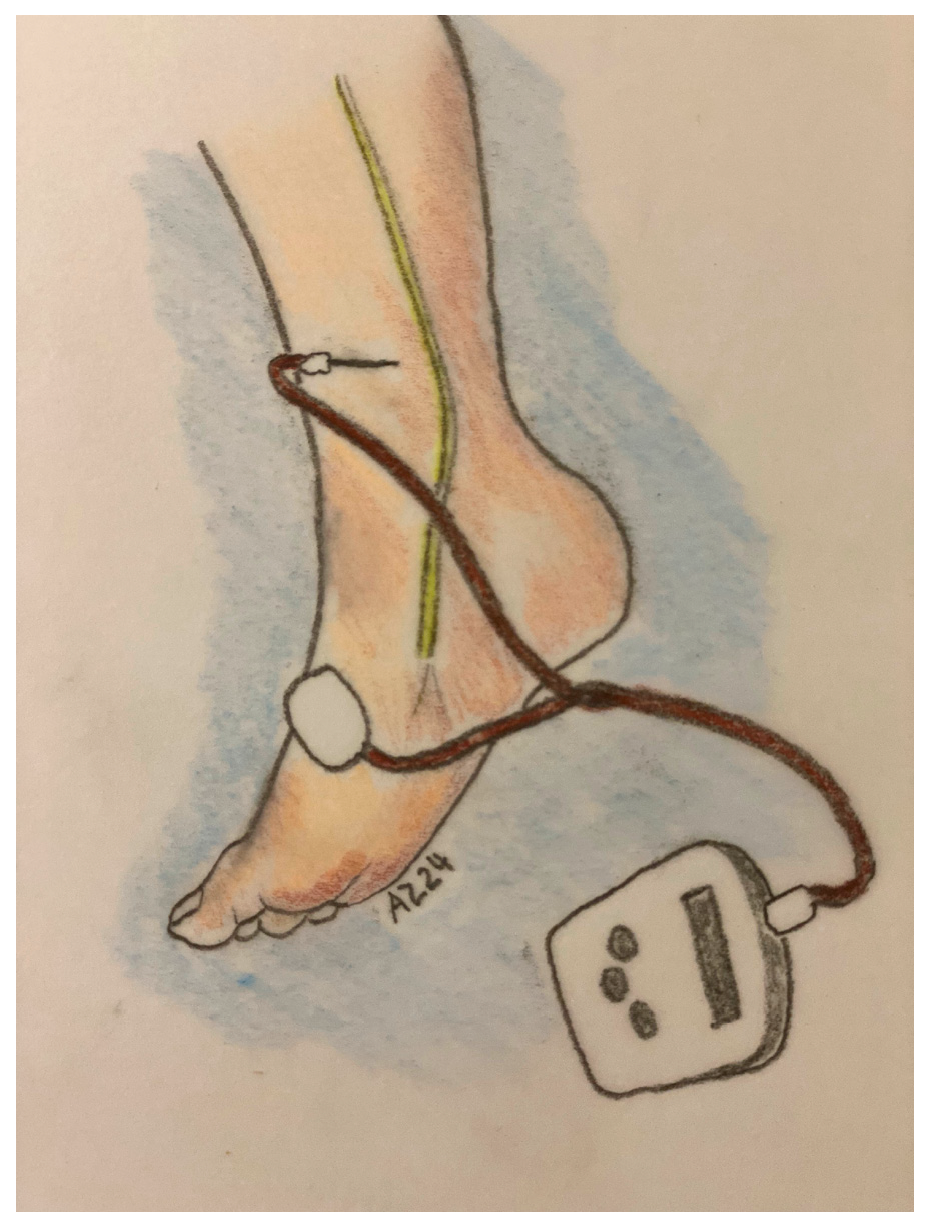
Figure 1.
PTNS technique, Stoller modification.
Figure 1.
PTNS technique, Stoller modification.
Table 1.
PTNS possible mechanisms and clinical effects.
Table 1.
PTNS possible mechanisms and clinical effects.
| Possible Mechanism | Clinical Effects |
|---|---|
| Impact of PTNS treatment on long-latency somatosensory evoked potentials (LL-SEPs) | Alterations in brain activity following PTNS relieving detrusor overactivity |
| PTNS effect on sacral and suprasacral centers involved in stimulus processing, potentially engaging relevant brain cortical regions. | Suppress detrusor activity by depolarizing somatic sacral and lumbar afferent fibers. |
| Influence on spinal cord function | Reduction in C-fos activity (a marker of neuronal metabolism) |
| Reduced mast cell population in the bladder | Reduced marker of cystitis-associated lower urinary tract dysfunction |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
[ad_2]